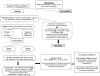
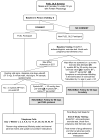
Design and rationale of the Fontan Udenafil Exercise Longitudinal (FUEL) trial
- PMID: 29910047
- PMCID: PMC6010060
- DOI: 10.1016/j.ahj.2018.03.015
Design and rationale of the Fontan Udenafil Exercise Longitudinal (FUEL) trial
Abstract
The Fontan operation creates a circulation characterized by elevated central venous pressure and low cardiac output. Over time, these characteristics result in a predictable and persistent decline in exercise performance that is associated with an increase in morbidity and mortality. A medical therapy that targets the abnormalities of the Fontan circulation might, therefore, be associated with improved outcomes. Udenafil, a phosphodiesterase type 5 inhibitor, has undergone phase I/II testing in adolescents who have had the Fontan operation and has been shown to be safe and well tolerated in the short term. However, there are no data regarding the long-term efficacy of udenafil in this population. The Fontan Udenafil Exercise Longitudinal (FUEL) Trial is a randomized, double-blind, placebo-controlled phase III clinical trial being conducted by the Pediatric Heart Network in collaboration with Mezzion Pharma Co, Ltd. This trial is designed to test the hypothesis that treatment with udenafil will lead to an improvement in exercise capacity in adolescents who have undergone the Fontan operation. A safety extension trial, the FUEL Open-Label Extension Trial (FUEL OLE), offers the opportunity for all FUEL subjects to obtain open-label udenafil for an additional 12 months following completion of FUEL, and evaluates the long-term safety and tolerability of this medication. This manuscript describes the rationale and study design for FUEL and FUEL OLE. Together, these trials provide an opportunity to better understand the role of medical management in the care of those who have undergone the Fontan operation.
Trial registration: ClinicalTrials.gov NCT02741115 NCT03013751.
Copyright © 2018 Elsevier Inc. All rights reserved.
References
-
- Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. The Journal of thoracic and cardiovascular surgery. 1973;66:613–21. - PubMed
-
- Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL109818/HL/NHLBI NIH HHS/United States
- U10 HL109741/HL/NHLBI NIH HHS/United States
- UG1 HL135646/HL/NHLBI NIH HHS/United States
- U10 HL109781/HL/NHLBI NIH HHS/United States
- U10 HL109743/HL/NHLBI NIH HHS/United States
- UG1 HL135682/HL/NHLBI NIH HHS/United States
- U10 HL068270/HL/NHLBI NIH HHS/United States
- U01 HL068270/HL/NHLBI NIH HHS/United States
- U10 HL109737/HL/NHLBI NIH HHS/United States
- U10 HL109777/HL/NHLBI NIH HHS/United States
- U10 HL109778/HL/NHLBI NIH HHS/United States
- UG1 HL135685/HL/NHLBI NIH HHS/United States
- U10 HL109816/HL/NHLBI NIH HHS/United States
- U10 HL109673/HL/NHLBI NIH HHS/United States
- UG1 HL135678/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical