Early Results of Fecal Microbial Transplantation Protocol Implementation at a Community-based University Hospital
- PMID: 29910564
- PMCID: PMC5987372
- DOI: 10.4103/jgid.jgid_145_17
Early Results of Fecal Microbial Transplantation Protocol Implementation at a Community-based University Hospital
Abstract
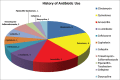
Introduction: Clostridium difficile (CD) is a serious and increasingly prevalent healthcare-associated infection. The pathogenesis of CD infection (CDI) involves the acquisition of CD with a concurrent disruption of the native gut flora. Antibiotics are a major risk although other contributing factors have also been identified. Clinical management combines discontinuation of the offending antibiotic, initiation of CD-specific antibiotic therapy, probiotic agent use, fecal microbiota transplantation (FMT), and surgery as the "last resort" option. The aim of this study is to review short-term clinical results following the implementation of FMT protocol (FMTP) at our community-based university hospital.
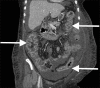
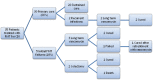
Methods: After obtaining Institutional Review Board and Infection Control Committee approvals, we implemented an institution-wide FMTP for patients diagnosed with CDI. Prospective tracking of all patients receiving FMT between July 1, 2015, and February 1, 2017, was conducted using REDCap™ electronic data capture system. According to the FMTP, indications for FMT included (a) three or more CDI recurrences, (b) two or more hospital admissions with severe CDI, or (c) first episode of complicated CDI (CCDI). Risk factors for initial infection and for treatment failure were assessed. Patients were followed for at least 3 months to monitor for cure/failure, relapse, and side effects. Frozen 250 mL FMT samples were acquired from OpenBiome (Somerville, MA, USA). After 4 h of thawing, the liquid suspension was applied using colonoscopy, beginning with terminal ileum and proceeding distally toward mid-transverse colon. Monitored clinical parameters included disease severity (Hines VA CDI Severity Score or HVCSS), concomitant medications, number of FMT treatments, non-FMT therapies, cure rates, and mortality. Descriptive statistics were utilized to outline the study results.
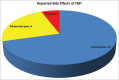
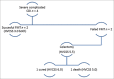
Results: A total of 35 patients (mean age 58.5 years, 69% female) were analyzed, with FMT-attributable primary cure achieved in 30/35 (86%) cases. Within this subgroup, 2/30 (6.7%) patients recurred and were subsequently cured with long-term oral vancomycin. Among five primary FMT failures (14% total sample), 3 (60%) achieved medical cure with long-term oral vancomycin therapy and 2 (40%) required colectomy. For the seven patients who either failed FMT or recurred, long-term vancomycin therapy was curative in all but two cases. For patients with severe CDI (HVCSS ≥3), primary and overall cure rates were 6/10 (60%) and 8/10 (80%), respectively. Patients with CCDI (n = 4) had higher HVCSS (4 vs. 3) and a mortality of 25%. Characteristics of patients who failed initial FMT included older age (70 vs. 57 years), female sex (80% vs. 67%), severe CDI (80% vs. 13%), and active opioid use during the initial infection (60% vs. 37%) and at the time of FMT (60% vs. 27%). The most commonly reported side effect of FMT was loose stools.
Conclusions: This pilot study supports the efficacy and safety of FMT administration for CDI in the setting of a community-based university hospital. Following FMTP implementation, primary (86%) and overall (94%) nonsurgical cure rates were similar to those reported in other studies. The potential role of opioids as a modulator of CDI warrants further clinical investigation.
Keywords: Clinical protocol implementation; Clostridium difficile; Clostridium difficile infection; fecal microbiota transplantation; infectious colitis.
Conflict of interest statement
There are no conflicts of interest.
Figures

References
-
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531–4. - PubMed
-
- McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–10. - PubMed
-
- Borgia G, Maraolo AE, Foggia M, Buonomo AR, Gentile I. Fecal microbiota transplantation for Clostridium difficile infection: Back to the future. Expert Opin Biol Ther. 2015;15:1001–14. - PubMed
-
- Keller JJ, Kuijper EJ. Treatment of recurrent and severe Clostridium difficile infection. Annu Rev Med. 2015;66:373–86. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

