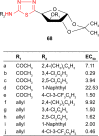
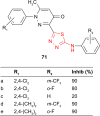
2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents
- PMID: 29910602
- PMCID: PMC5987787
- DOI: 10.2147/DDDT.S155958
2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents
Abstract
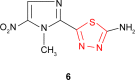
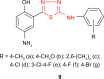
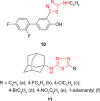
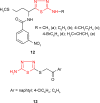
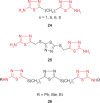
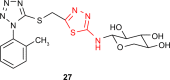
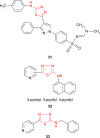
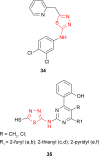
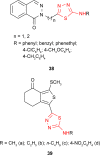
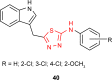
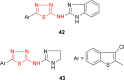
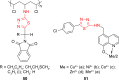
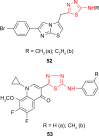
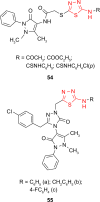
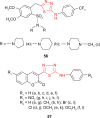
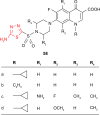
Pathogenic microorganisms are causative agents for different types of serious and even lethal infectious diseases. Despite advancements in medication, bacterial and fungal infections continue to be a growing problem in health care. As more and more bacteria become resistant to antibiotics used in therapy and an increasing number of invasive fungal species become resistant to current antifungal medications, there is considerable interest in the development of new compounds with antimicrobial activity. The compounds containing a heterocyclic ring play an important role among organic compounds with biological activity used as drugs in human and veterinary medicine or as insecticides and pesticides in agriculture. Thiadiazoles belong to the classes of nitrogen-sulfur heterocycles with extensive application as structural units of biologically active molecules and as useful intermediates in medicinal chemistry. The potency of the thiadiazole nucleus is demonstrated by the drugs currently used. 1,3,4-Thiadiazoles and some of their derivatives are extensively studied because of their broad spectrum of pharmacological activities. The aim of this review was to highlight the main antimicrobial properties exhibited by derivatives possessing 2-amino-1,3,4-thiadiazole moiety. Many of the reported 2-amino-1,3,4-thiadiazole derivatives can be considered as lead compounds for drug synthesis, and several of them have demonstrated higher antimicrobial activity in comparison to standard drugs. Furthermore, taking into account the reactivity of the amine group in the derivatization process, 2-amino-1,3,4-thiadiazole moiety may be a good scaffold for future pharmacologically active 1,3,4-thiadiazole derivatives.
Keywords: 2-amino-1,3,4-thiadiazole; antibacterial activity; antifungal activity; antimicrobial activity; antitubercular activity; minimum inhibitory concentration.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Wermuth CG, Ciapetti P, Giethlen B, Bazzini P. Bioisosterism. In: Taylor JB, Triggle DJ, editors. Comprehensive Medicinal Chemistry. Vol. 2. Amsterdam: Elsevier; 2007. pp. 649–711.
-
- Holla BS, Poorjary KN, Rao BS, Shivananda MK. New bis-aminomercaptotriazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur J Med Chem. 2002;37:511–517. - PubMed
-
- Yousif E, Majeed A, Al-Sammarrae K, Salih N, Salimon J, Abdullah B. Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arabian J Chem. 2013;5(S2) doi: 10.1016/j.arabjc.2013.06.006. - DOI
-
- Li Y, Geng J, Liu Y, Yu S, Zhao G. Thiadiazole – a promising structure in medicinal chemistry. ChemMedChem. 2013;8(1):27–41. - PubMed
-
- Wermuth CG, Aldous D, Raboisson P, Rognan D. The Practice of Medicinal Chemistry. 4th ed. London: Academic Press, Elsevier; 2015. p. 196.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials