Hybridization and antibiotic synergism as a tool for reducing the cytotoxicity of antimicrobial peptides
- PMID: 29910626
- PMCID: PMC5987794
- DOI: 10.2147/IDR.S166236
Hybridization and antibiotic synergism as a tool for reducing the cytotoxicity of antimicrobial peptides
Abstract
Introduction: As the development of new antimicrobial agents faces a historical decline, the issue of bacterial drug resistance has become a serious dilemma that threatens the human population worldwide. Antimicrobial peptides (AMPs) represent an attractive and a promising class of antimicrobial agents.
Aim: The hybridization of AMPs aimed at merging two individual active fragments of native peptides to generate a new AMP with altered physicochemical properties that translate into an enhanced safety profile.
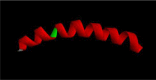
Materials and methods: In this study, we have rationally designed a new hybrid peptide via combining two individual α-helical fragments of both BMAP-27 and OP-145. The resultant peptide, was evaluated for its antimicrobial and antibiofilm activity against a range of microbial strains. The resultant peptide was also evaluated for its toxicity against mammalian cells using hemolytic and anti proliferative assays.
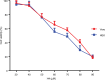
Results: The antimicrobial activity of H4 revealed that the peptide is displaying a broad spectrum of activity against both Gram-positive and Gram-negative bacteria including standard and multidrug-resistant bacterial strains in the range of 2.5-25 μM. The new hybrid peptide displayed potent activity in eradicating biofilm-forming cells, and the reported minimum biofilm eradication concentrations were equal to the minimum inhibitory concentration values reported for planktonic cells. Additionally, H4 exhibited reduced toxicity profiles against eukaryotic cells. Combining H4 peptide with conventional antibiotics has led to a dramatic enhancement of the antimicrobial activity of both agents with synergistic or additive outcomes.
Conclusion: Overall, this study indicates the success of both the hybridization and synergism strategy in developing AMPs as potential antimicrobial therapeutics with reduced toxicity profiles that could be efficiently employed to eradicate resistant bacterial strains and enhance the selectivity and toxicity profiles of native AMPs.
Keywords: antibiotic synergism; antimicrobial peptides; antimicrobial resistance; biofilms; peptide hybridization.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Luepke KH, Suda KJ, Boucher H, et al. Past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy. 2017;37(1):71–84. - PubMed
-
- Kang SJ, Park SJ, Mishig-Ochir T, Lee BJ. Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther. 2014;12(12):1477–1486. - PubMed
-
- Sun E, Belanger CR, Haney EF, Hancock REW. Peptide Applications in Biomedicine, Biotechnology and Bioengineering. Woodhead Publishing; 2018. Host defense (antimicrobial) peptides; pp. 253–285.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

