Functional Consequences of the Postnatal Switch From Neonatal to Mutant Adult Glycine Receptor α1 Subunits in the Shaky Mouse Model of Startle Disease
- PMID: 29910711
- PMCID: PMC5992992
- DOI: 10.3389/fnmol.2018.00167
Functional Consequences of the Postnatal Switch From Neonatal to Mutant Adult Glycine Receptor α1 Subunits in the Shaky Mouse Model of Startle Disease
Abstract
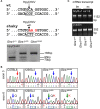
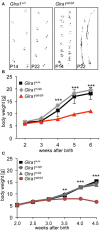
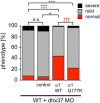
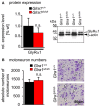
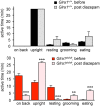
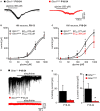
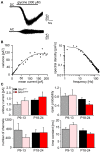
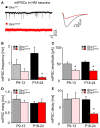
Mutations in GlyR α1 or β subunit genes in humans and rodents lead to severe startle disease characterized by rigidity, massive stiffness and excessive startle responses upon unexpected tactile or acoustic stimuli. The recently characterized startle disease mouse mutant shaky carries a missense mutation (Q177K) in the β8-β9 loop within the large extracellular N-terminal domain of the GlyR α1 subunit. This results in a disrupted hydrogen bond network around K177 and faster GlyR decay times. Symptoms in mice start at postnatal day 14 and increase until premature death of homozygous shaky mice around 4-6 weeks after birth. Here we investigate the in vivo functional effects of the Q177K mutation using behavioral analysis coupled to protein biochemistry and functional assays. Western blot analysis revealed GlyR α1 subunit expression in wild-type and shaky animals around postnatal day 7, a week before symptoms in mutant mice become obvious. Before 2 weeks of age, homozygous shaky mice appeared healthy and showed no changes in body weight. However, analysis of gait and hind-limb clasping revealed that motor coordination was already impaired. Motor coordination and the activity pattern at P28 improved significantly upon diazepam treatment, a pharmacotherapy used in human startle disease. To investigate whether functional deficits in glycinergic neurotransmission are present prior to phenotypic onset, we performed whole-cell recordings from hypoglossal motoneurons (HMs) in brain stem slices from wild-type and shaky mice at different postnatal stages. Shaky homozygotes showed a decline in mIPSC amplitude and frequency at P9-P13, progressing to significant reductions in mIPSC amplitude and decay time at P18-24 compared to wild-type littermates. Extrasynaptic GlyRs recorded by bath-application of glycine also revealed reduced current amplitudes in shaky mice compared to wild-type neurons, suggesting that presynaptic GlyR function is also impaired. Thus, a distinct, but behaviorally ineffective impairment of glycinergic synapses precedes the symptoms onset in shaky mice. These findings extend our current knowledge on startle disease in the shaky mouse model in that they demonstrate how the progression of GlyR dysfunction causes, with a delay of about 1 week, the appearance of disease symptoms.
Keywords: fast decay; glycine receptor; mouse model; shaky; startle disease; β8-β9 loop.
Figures

Similar articles
-
Role of the Glycine Receptor β Subunit in Synaptic Localization and Pathogenicity in Severe Startle Disease.J Neurosci. 2024 Jan 10;44(2):e0837232023. doi: 10.1523/JNEUROSCI.0837-23.2023. J Neurosci. 2024. PMID: 37963764 Free PMC article.
-
Disruption of a Structurally Important Extracellular Element in the Glycine Receptor Leads to Decreased Synaptic Integration and Signaling Resulting in Severe Startle Disease.J Neurosci. 2017 Aug 16;37(33):7948-7961. doi: 10.1523/JNEUROSCI.0009-17.2017. Epub 2017 Jul 19. J Neurosci. 2017. PMID: 28724750 Free PMC article.
-
The GlyR Extracellular β8-β9 Loop - A Functional Determinant of Agonist Potency.Front Mol Neurosci. 2017 Oct 9;10:322. doi: 10.3389/fnmol.2017.00322. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29062270 Free PMC article.
-
Impaired Glycine Receptor Trafficking in Neurological Diseases.Front Mol Neurosci. 2018 Aug 21;11:291. doi: 10.3389/fnmol.2018.00291. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30186111 Free PMC article. Review.
-
The genetics of hyperekplexia: more than startle!Trends Genet. 2008 Sep;24(9):439-47. doi: 10.1016/j.tig.2008.06.005. Epub 2008 Aug 15. Trends Genet. 2008. PMID: 18707791 Review.
Cited by
-
Comprehensive behavioral analyses of mice with a glycine receptor alpha 4 deficiency.Mol Brain. 2023 May 22;16(1):44. doi: 10.1186/s13041-023-01033-x. Mol Brain. 2023. PMID: 37217969 Free PMC article.
-
Contribution of GlyR α3 Subunits to the Sensitivity and Effect of Ethanol in the Nucleus Accumbens.Front Mol Neurosci. 2021 Oct 22;14:756607. doi: 10.3389/fnmol.2021.756607. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34744627 Free PMC article.
-
Role of the Glycine Receptor β Subunit in Synaptic Localization and Pathogenicity in Severe Startle Disease.J Neurosci. 2024 Jan 10;44(2):e0837232023. doi: 10.1523/JNEUROSCI.0837-23.2023. J Neurosci. 2024. PMID: 37963764 Free PMC article.
-
Spinal Cord Neuronal Network Formation in a 3D Printed Reinforced Matrix-A Model System to Study Disease Mechanisms.Adv Healthc Mater. 2021 Oct;10(19):e2100830. doi: 10.1002/adhm.202100830. Epub 2021 Aug 5. Adv Healthc Mater. 2021. PMID: 34350717 Free PMC article.
-
Startle Disease: New Molecular Insights into an Old Neurological Disorder.Neuroscientist. 2023 Dec;29(6):767-781. doi: 10.1177/10738584221104724. Epub 2022 Jun 25. Neuroscientist. 2023. PMID: 35754344 Free PMC article. Review.
References
-
- Becker K., Braune M., Benderska N., Buratti E., Baralle F., Villmann C., et al. . (2012). A retroelement modifies pre-mRNA splicing: the murine Glrbspa allele is a splicing signal polymorphism amplified by long interspersed nuclear element insertion. J. Biol. Chem. 287, 31185–31194. 10.1074/jbc.M112.375691 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

