Insights Into Protein S-Palmitoylation in Synaptic Plasticity and Neurological Disorders: Potential and Limitations of Methods for Detection and Analysis
- PMID: 29910712
- PMCID: PMC5992399
- DOI: 10.3389/fnmol.2018.00175
Insights Into Protein S-Palmitoylation in Synaptic Plasticity and Neurological Disorders: Potential and Limitations of Methods for Detection and Analysis
Abstract
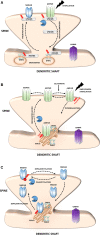
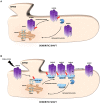
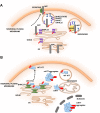
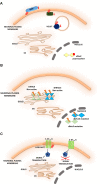
S-palmitoylation (S-PALM) is a lipid modification that involves the linkage of a fatty acid chain to cysteine residues of the substrate protein. This common posttranslational modification (PTM) is unique among other lipid modifications because of its reversibility. Hence, like phosphorylation or ubiquitination, it can act as a switch that modulates various important physiological pathways within the cell. Numerous studies revealed that S-PALM plays a crucial role in protein trafficking and function throughout the nervous system. Notably, the dynamic turnover of palmitate on proteins at the synapse may provide a key mechanism for rapidly changing synaptic strength. Indeed, palmitate cycling on postsynaptic density-95 (PSD-95), the major postsynaptic density protein at excitatory synapses, regulates the number of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and thus affects synaptic transmission. Accumulating evidence suggests a relationship between impairments in S-PALM and severe neurological disorders. Therefore, determining the precise levels of S-PALM may be essential for understanding the ways in which this PTM is regulated in the brain and controls synaptic dynamics. Protein S-PALM can be characterized using metabolic labeling methods and biochemical tools. Both approaches are discussed herein in the context of specific methods and their advantages and disadvantages. This review clearly shows progress in the field, which has led to the development of new, more sensitive techniques that enable the detection of palmitoylated proteins and allow predictions of potential palmitate binding sites. Unfortunately, one significant limitation of these approaches continues to be the inability to use them in living cells.
Keywords: S-palmitoylation; biochemical methods; metabolic labeling; neurodegenerative diseases; synapse; synaptic plasticity.
Figures



Similar articles
-
Stress-induced Changes in the S-palmitoylation and S-nitrosylation of Synaptic Proteins.Mol Cell Proteomics. 2019 Oct;18(10):1916-1938. doi: 10.1074/mcp.RA119.001581. Epub 2019 Jul 16. Mol Cell Proteomics. 2019. PMID: 31311849 Free PMC article.
-
Protein Palmitoylation by DHHC Protein Family.In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 5. In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 5. PMID: 21204476 Free Books & Documents. Review.
-
Identification of PSD-95 Depalmitoylating Enzymes.J Neurosci. 2016 Jun 15;36(24):6431-44. doi: 10.1523/JNEUROSCI.0419-16.2016. J Neurosci. 2016. PMID: 27307232 Free PMC article.
-
Deficiency of AMPAR-Palmitoylation Aggravates Seizure Susceptibility.J Neurosci. 2018 Nov 21;38(47):10220-10235. doi: 10.1523/JNEUROSCI.1590-18.2018. Epub 2018 Oct 24. J Neurosci. 2018. PMID: 30355633 Free PMC article.
-
Palmitoylation-mediated synaptic regulation of AMPA receptor trafficking and function.Arch Pharm Res. 2019 May;42(5):426-435. doi: 10.1007/s12272-019-01134-z. Epub 2019 Mar 5. Arch Pharm Res. 2019. PMID: 30838509 Free PMC article. Review.
Cited by
-
Gynura divaricata Water Extract Presented the Possibility to Enhance Neuronal Regeneration.Evid Based Complement Alternat Med. 2021 Feb 17;2021:8818618. doi: 10.1155/2021/8818618. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33680064 Free PMC article.
-
ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5.Nat Chem Biol. 2019 Dec;15(12):1232-1240. doi: 10.1038/s41589-019-0399-y. Epub 2019 Nov 18. Nat Chem Biol. 2019. PMID: 31740833 Free PMC article.
-
Selective vulnerability of tripartite synapses in amyotrophic lateral sclerosis.Acta Neuropathol. 2022 Apr;143(4):471-486. doi: 10.1007/s00401-022-02412-9. Epub 2022 Mar 19. Acta Neuropathol. 2022. PMID: 35305541 Free PMC article.
-
Nutrient-Dependent Changes of Protein Palmitoylation: Impact on Nuclear Enzymes and Regulation of Gene Expression.Int J Mol Sci. 2018 Nov 30;19(12):3820. doi: 10.3390/ijms19123820. Int J Mol Sci. 2018. PMID: 30513609 Free PMC article. Review.
-
The Agro-Physiological and Phytochemical Potential of Quinoa (Chenopodium quinoa Willd.) Under Saline Stress: A Comprehensive Investigation of Nutritional Properties and Antioxidant Activities.Plants (Basel). 2025 May 15;14(10):1482. doi: 10.3390/plants14101482. Plants (Basel). 2025. PMID: 40431046 Free PMC article.
References
-
- Andrew R. J., Fernandez C. G., Stanley M., Jiang H., Nguyen P., Rice R. C., et al. (2017). Lack of BACE1 S-palmitoylation reduces amyloid burden and mitigates memory deficits in transgenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 114 E9665–E9674. 10.1073/pnas.1708568114 - DOI - PMC - PubMed
-
- Ardiles A. O., Tapia-Rojas C. C., Mandal M., Alexandre F., Kirkwood A., Inestrosa N. C., et al. (2012). Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 109 13835–13840. 10.1073/pnas.1201209109 - DOI - PMC - PubMed
-
- Battaglia S., Renner M., Russeau M., Côme E., Tyagarajan S., Lévi S., et al. (2018). Activity-dependent inhibitory synapse scaling is determined by gephyrin phosphorylation and subsequent regulation of GABAA receptor diffusion. eNeuro 5:ENEURO.203–ENEURO.217. 10.1523/ENEURO.0203-17.2017 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

