Squeezing for Life - Properties of Red Blood Cell Deformability
- PMID: 29910743
- PMCID: PMC5992676
- DOI: 10.3389/fphys.2018.00656
Squeezing for Life - Properties of Red Blood Cell Deformability
Abstract
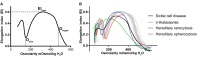
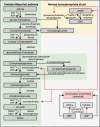
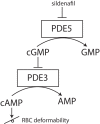
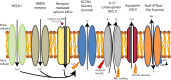
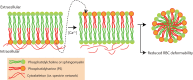
Deformability is an essential feature of blood cells (RBCs) that enables them to travel through even the smallest capillaries of the human body. Deformability is a function of (i) structural elements of cytoskeletal proteins, (ii) processes controlling intracellular ion and water handling and (iii) membrane surface-to-volume ratio. All these factors may be altered in various forms of hereditary hemolytic anemia, such as sickle cell disease, thalassemia, hereditary spherocytosis and hereditary xerocytosis. Although mutations are known as the primary causes of these congenital anemias, little is known about the resulting secondary processes that affect RBC deformability (such as secondary changes in RBC hydration, membrane protein phosphorylation, and RBC vesiculation). These secondary processes could, however, play an important role in the premature removal of the aberrant RBCs by the spleen. Altered RBC deformability could contribute to disease pathophysiology in various disorders of the RBC. Here we review the current knowledge on RBC deformability in different forms of hereditary hemolytic anemia and describe secondary mechanisms involved in RBC deformability.
Keywords: deformability; enzymopathies; hemolysis; hereditary spherocytosis; hydration; sickle cell anemia; thalassemia; vesiculation.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

