Extracellular Vesicles in Chagas Disease: A New Passenger for an Old Disease
- PMID: 29910793
- PMCID: PMC5992290
- DOI: 10.3389/fmicb.2018.01190
Extracellular Vesicles in Chagas Disease: A New Passenger for an Old Disease
Abstract
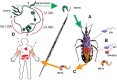
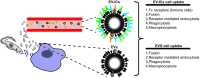
Extracellular vesicles (EVs) are small lipid vesicles released by prokaryotic and eukaryotic cells containing nucleic acids, proteins, and small metabolites essential for cellular communication. Depending on the targeted cell, EVs can act either locally or in distant tissues in a paracrine or endocrine cell signaling manner. Released EVs from virus-infected cells, bacteria, fungi, or parasites have been demonstrated to perform a pivotal role in a myriad of biochemical changes occurring in the host and pathogen, including the modulation the immune system. In the past few years, the biology of Trypanosoma cruzi EVs, as well as their role in innate immunity evasion, has been started to be unveiled. This review article will present findings on and provide a coherent understanding of the currently known mechanisms of action of T. cruzi-EVs and hypothesize the implication of these parasite components during the acute and chronic phases of Chagas disease.
Keywords: Leishmania spp.; Trypanosoma brucei; Trypanosoma cruzi; ectosome; exosome; kinetoplastids; microvesicle; pathogen.
Figures

References
-
- Bayer-Santos E., Aguilar-Bonavides C., Rodrigues S. P., Cordero E. M., Marques A. F., Varela-Ramirez A., et al. (2013). Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. J. Proteome Res. 12 883–897. 10.1021/pr300947g - DOI - PubMed
-
- Bermejo D. A., Amezcua Vesely M. C., Khan M., Acosta Rodríguez E. V., Montes C. L., Merino M. C., et al. (2011). Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B-cell response which is a high source of non-parasite-specific antibodies: B-cell response to T. cruzi. Immunology 132 123–133. 10.1111/j.1365-2567.2010.03347.x - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

