C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor
- PMID: 29910808
- PMCID: PMC5992430
- DOI: 10.3389/fimmu.2018.01167
C-Reactive Protein (CRP) and Leptin Receptor in Obesity: Binding of Monomeric CRP to Leptin Receptor
Abstract
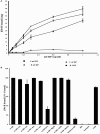
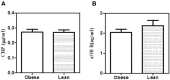
While leptin deficiency or dysfunction leads to morbid obesity, obese subjects are characterized paradoxically by hyperleptinemia indicating lack of response to leptin. C-reactive protein (CRP) has been suggested to be a key plasma protein that could bind to leptin. To examine whether CRP interferes with leptin action, mediated through its cell surface receptor, docking studies of CRP with the extracellular domain of the leptin receptor were done employing bioinformatics tools. Monomeric CRP docked with better Z-rank score and more non-bond interactions than pentameric CRP at the CRH2-FNIII domain proximal to the cell membrane, distinct from the leptin-docking site. Interaction of CRP with leptin receptor was validated by solid phase binding assay and co-immunoprecipitation of CRP and soluble leptin receptor (sOb R) from human plasma. Analysis of the serum levels of leptin, CRP, and sOb R by ELISA showed that CRP levels were significantly elevated (p < 0.0001) in non-morbid obese subjects (n = 42) compared to lean subjects (n = 32) and correlated positively with body mass index (BMI) (r = 0.74, p < 0.0001) and leptin (r = 0.8, p < 0.0001); levels of sOb R were significantly low in obese subjects (p < 0.001) and showed a negative correlation with BMI (r = -0.26, p < 0.05) and leptin (r = -0.23, p < 0.05) indicating a minimal role for sOb R in sequestering leptin.
Keywords: leptin; leptin receptor; monomeric C-reactive protein; obesity; protein–protein docking.
Figures


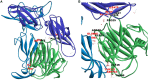



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

