Effector T Helper Cell Subsets in Inflammatory Bowel Diseases
- PMID: 29910812
- PMCID: PMC5992276
- DOI: 10.3389/fimmu.2018.01212
Effector T Helper Cell Subsets in Inflammatory Bowel Diseases
Abstract
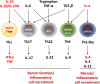
The gastrointestinal tract is a site of high immune challenge, as it must maintain a delicate balance between tolerating luminal contents and generating an immune response toward pathogens. CD4+ T cells are key in mediating the host protective and homeostatic responses. Yet, CD4+ T cells are also known to be the main drivers of inflammatory bowel disease (IBD) when this balance is perturbed. Many subsets of CD4+ T cells have been identified as players in perpetuating chronic intestinal inflammation. Over the last few decades, understanding of how each subset of Th cells plays a role has dramatically increased. Simultaneously, this has allowed development of therapeutic innovation targeting specific molecules rather than broad immunosuppressive agents. Here, we review the emerging evidence of how each subset functions in promoting and sustaining the chronic inflammation that characterizes IBD.
Keywords: Crohn’s disease; T helper cells; inflammatory bowel disease; inflammatory cytokines; transcription factors; ulcerative colitis.
Figures
References
-
- Greenwald B, James SP. Long-term HIV infection with Crohn’s disease. Am J Gastroenterol (1995) 90(1):167–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

