Discovery of new mutually orthogonal bioorthogonal cycloaddition pairs through computational screening
- PMID: 29910881
- PMCID: PMC4763938
- DOI: 10.1039/c5sc03259h
Discovery of new mutually orthogonal bioorthogonal cycloaddition pairs through computational screening
Abstract
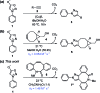
Density functional theory (DFT) calculations and experiments in tandem led to discoveries of new reactivities and selectivities involving bioorthogonal sydnone cycloadditions. Dibenzocyclooctyne derivatives (DIBAC and BARAC) were identified to be especially reactive dipolarophiles, which undergo the (3 + 2) cycloadditions with N-phenyl sydnone with the rate constant of up to 1.46 M-1 s-1. Most significantly, the sydnone-dibenzocyclooctyne and norbornene-tetrazine cycloadditions were predicted to be mutually orthogonal. This was validated experimentally and used for highly selective fluorescence labeling of two proteins simultaneously.
Figures




References
-
- Saxon E., Bertozzi C. R. Science. 2000;287:2007–2010. - PubMed
-
- Baskin J. M., Prescher J. A., Laughlin S. T., Agard N. J., Chang P. V., Miller I. A., Lo A., Codelli J. A., Bertozzi C. R. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16793–16797. - PMC - PubMed
- Codelli J. A., Baskin J. M., Agard N. J., Bertozzi C. R. J. Am. Chem. Soc. 2008;130:11486–11493. - PMC - PubMed
- Ning X., Guo J., Wolfert M. A., Boons G. J. Angew. Chem., Int. Ed. 2008;47:2253–2255. - PMC - PubMed
- Debets M. F., van Berkel S. S., Schoffelen S., Rutjes F. P. J. T., van Hest J. C. M., van Delft F. L. Chem. Commun. 2010;46:97–99. - PubMed
- Jewett J. C., Sletten E. M., Bertozzi C. R. J. Am. Chem. Soc. 2010;132:3688–3690. - PMC - PubMed
- Dommerholt J., Schmidt S., Temming R., Hendriks L. J., Rutjes F. P., van Hest J., Lefeber D. J., Friedl P., van Delft F. L. Angew. Chem., Int. Ed. 2010;49:9422–9425. - PMC - PubMed
- Plass T., Milles S., Koehler C., Schultz C., Lemke E. A. Angew. Chem., Int. Ed. 2011;50:3878–3881. - PMC - PubMed
- Debets M. F., Van Berkel S. S., Dommerholt J., Dirks A. J., Rutjes F. P., van Delft F. L. Acc. Chem. Res. 2011;44:805–815. - PubMed
- Lim R. K., Lin Q. Acc. Chem. Res. 2011;44:828–839. - PMC - PubMed
- Yu Z., Pan Y., Wang Z., Wang J., Lin Q. Angew. Chem., Int. Ed. 2012;51:10600–10604. - PMC - PubMed
- Yu Z., Ohulchanskyy T. Y., An P., Prasad P. N., Lin Q. J. Am. Chem. Soc. 2013;135:16766–16769. - PMC - PubMed
- Yu Z., Lin Q. J. Am. Chem. Soc. 2014;136:4153–4156. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

