β-Arylation of oxime ethers using diaryliodonium salts through activation of inert C(sp)-H bonds using a palladium catalyst
- PMID: 29910895
- PMCID: PMC5975923
- DOI: 10.1039/c5sc03903g
β-Arylation of oxime ethers using diaryliodonium salts through activation of inert C(sp)-H bonds using a palladium catalyst
Abstract
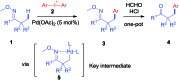
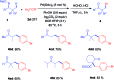
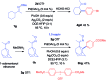
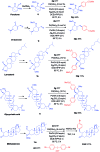
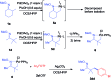
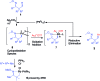
An efficient method of selective β-arylation of oxime ethers was realized by using a palladium catalyst with diaryliodonium salts as the key arylation reagents. The reaction proceeded smoothly through the activation of inert C(sp3)-H bonds to give corresponding ketones and aldehydes. This convenient procedure can be successfully applied to construct new C(sp3)-C(sp2) bonds on a number of complex molecules derived from natural products and thus serves as a practical synthetic tool for direct late-stage C(sp3)-H functionalization.
Figures

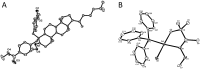
References
-
- Godula K., Sames D. Science. 2006;312:67. - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147. - PMC - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902. - PubMed
- Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960. - PubMed
- Arockiam P. B., Bruneau C., Dixneuf P. H. Chem. Rev. 2012;112:5879. - PubMed
- Rouquet G., Chatani N. Angew. Chem., Int. Ed. 2013;52:11726. - PubMed
-
-
For selective examples, see:
- Culkin D. A., Hartwig J. F. Acc. Chem. Res. 2003;36:234. - PubMed
- Davies H. M. L. Angew. Chem., Int. Ed. 2006;45:6422. - PubMed
- Shi B.-F., Maugel N., Zhang Y.-H., Yu J.-Q. Angew. Chem., Int. Ed. 2008;47:4882. - PubMed
- Godula K., Sames D. Science. 2009;312:67. - PubMed
- Phipps R. J., Gaunt M. J. Science. 2009;323:1593. - PubMed
- Doyle M. P., Duffy R., Ratnikov M., Zhou L. Chem. Rev. 2010;110:704. - PubMed
- Sun C.-L., Li B.-J., Shi Z.-J. Chem. Rev. 2011;111:1293. - PubMed
- Li H., Li B.-J., Shi Z.-J. Catal. Sci. Technol. 2011;1:191.
- Shin K., Park S.-W., Chang S. J. Am. Chem. Soc. 2015;137:8584. - PubMed
- Stokes B. J., Liao L.-Y., Andrade A. M., Wang Q.-F., Sigman M. S. Org. Lett. 2014;16:4666. - PMC - PubMed
- Mei T.-S., Harshkumar H. P., Sigman M. S. Nature. 2014;508:340. - PMC - PubMed
- Werner E. W., Mei T.-S., Burckle A. J., Sigman M. S. Science. 2012;338:1455. - PMC - PubMed
- Cahard E., Male H. P. J., Tissot M., Gaunt M. J. J. Am. Chem. Soc. 2015;137:7986. - PMC - PubMed
-
-
- Jørgensen M., Lee S., Liu X.-X., Wolkowski J. P., Hartwig J. F. J. Am. Chem. Soc. 2002;124:12557. - PubMed
- Zaitsev V. G., Shabashov D., Daugulis O. J. Am. Chem. Soc. 2005;127:13154. - PubMed
- Feng Y., Wang Y., Landgraf B., Liu S., Chen G. Org. Lett. 2010;12:3414. - PubMed
- Renaudat A., Jean-Gerard L., Jazzar R., Kefalidis C. E., Clot E., Baudoin O. Angew. Chem., Int. Ed. 2010;49:7261. - PubMed
- Wasa M., Engle K. M., Lin D. W., Yoo E. J., Yu J.-Q. J. Am. Chem. Soc. 2011;133:19598. - PMC - PubMed
- Aspin S., Goutierre A. S., Larini P., Jazzar R., Baudoin O. Angew. Chem., Int. Ed. 2012;51:10808. - PubMed
- Shang R., Ilies L., Matsumoto A., Nakamura E. J. J. Am. Chem. Soc. 2013;135:6030. - PubMed
- Pan F., Shen P.-X., Zhang L.-S., Wang X., Shi Z.-J. Org. Lett. 2013;15:4758. - PubMed
-
- Reddy B. V. S., Reddy L. R., Corey E. J. Org. Lett. 2006;8:3391. - PubMed
- Rodriguez N., Romero-Revilla J. A., Fernandez-Ibanez M. A., Carretero J. C. Chem. Sci. 2013;4:175.
- Chan K. S. L., Wasa M., Chu L., Laforteza B. N., Miura M., Yu J.-Q. Nat. Chem. 2014;6:146. - PMC - PubMed
- Chan K. S. L., Fu H.-Y., Yu J.-Q. J. Am. Chem. Soc. 2015;137:2042. - PMC - PubMed
- Millet A., Dailler D., Larini P., Baoudoin O. Angew. Chem., Int. Ed. 2014;53:2678. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

