Quantitative model for rationalizing solvent effect in noncovalent CH-Aryl interactions
- PMID: 29910898
- PMCID: PMC5975927
- DOI: 10.1039/c5sc03550c
Quantitative model for rationalizing solvent effect in noncovalent CH-Aryl interactions
Abstract
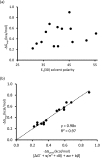
The strength of CH-aryl interactions (ΔG) in 14 solvents was determined via the conformational analysis of a molecular torsion balance. The molecular balance adopted folded and unfolded conformers in which the ratio of the conformers in solution provided a quantitative measure of ΔG as a function of solvation. While a single empirical solvent parameter based on solvent polarity failed to explain solvent effect in the molecular balance, it is shown that these ΔG values can be correlated through a multiparameter linear solvation energy relationship (LSER) using the equation introduced by Kamlet and Taft. The resulting LSER equation [ΔG = -0.24 + 0.23α - 0.68β - 0.1π* + 0.09δ]-expresses ΔG as a function of Kamlet-Taft solvent parameters-revealed that specific solvent effects (α and β) are mainly responsible for "tipping" the molecular balance in favour of one conformer over the other, where α represents a solvents' hydrogen-bond acidity and β represents a solvents' hydrogen-bond basicity. Furthermore, using extrapolated data (α and β) and the known π* value for the gas phase, the LSER equation predicted ΔG in the gas phase to be -0.31 kcal mol-1, which agrees with -0.35 kcal mol-1 estimated from DFT-D calculations.
Figures




References
-
- McGaughey G. B., Gagne M., Rappe A. K. J. Biol. Chem. 1998;273:15458–15463. - PubMed
-
- Muller-Dethlefs K., Hobza P. Chem. Rev. 2000;100:143–167. - PubMed
-
- Meyer E. A., Castellano R. K., Diederich F. Angew. Chem., Int. Ed. 2003;42:1210–1250. - PubMed
-
- Tsuzuki S., Fujii A. PCCP Phys. Chem. Chem. Phys. 2008;10:2584–2594. - PubMed
-
- Tsuzuki S., Honda K., Uchimaru T., Mikami M., Fujii A. J. Phys. Chem. A. 2006;110:10163–10168. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

