A unique approach toward near-infrared fluorescent probes for bioimaging with remarkably enhanced contrast
- PMID: 29910917
- PMCID: PMC5977507
- DOI: 10.1039/c5sc04014k
A unique approach toward near-infrared fluorescent probes for bioimaging with remarkably enhanced contrast
Abstract
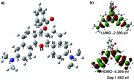
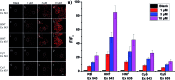
Near-infrared (NIR) fluorescent probes are attractive molecular tools for bioimaging because of their low autofluorescence interference, deep tissue penetration, and minimal damage to sample. However, most previously reported NIR probes exhibit small Stokes shift, typically less than 30 nm, and low fluorescence quantum yield, strictly limited contrast and spatial resolution for bioimaging. Herein, by expanding the π-conjugated system of rhodamine B, while, at the same time, keeping its rigid and planar structure, we reported an efficient NIR dye, HN7, with large stokes shift of 73 nm and fluorescence quantum yield as high as 0.72 in ethanol, values superior to those of such traditional cyanine NIR dyes as Cy5. Using HN7, living cells, tissues and mice were imaged, and the results showed significantly enhanced contrast, improved spatial resolution, and satisfactory tissue imaging depth when compared to Cy5. Moreover, the nonfluorescent spirocyclic structure of rhodamine B is an inherent component of HN7; therefore, our strategy provided a universal platform for the design of efficient NIR turn-on bioimaging probes for various targets. As a proof-of-concept, two different NIR probes, HN7-N2 and HN7-S for NO and Hg2+, respectively, were designed, synthesized, and successfully applied for the imaging of NO and Hg2+ in living cells, tissues and mice, respectively, demonstrating the potential bioimaging applications of the new probes. In sum, this new type of dye may present new avenues for the development of efficient NIR fluorescent probes for contrast-enhanced imaging in biological applications.
Figures






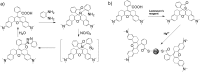




Similar articles
-
Analogs of Changsha near-infrared dyes with large Stokes Shifts for bioimaging.Biomaterials. 2013 Dec;34(37):9566-71. doi: 10.1016/j.biomaterials.2013.08.081. Epub 2013 Sep 17. Biomaterials. 2013. PMID: 24054843
-
The latest developments of near-infrared fluorescent probes from NIR-I to NIR-II for bioimaging.Anal Sci. 2025 Jun;41(6):737-757. doi: 10.1007/s44211-025-00735-7. Epub 2025 Feb 28. Anal Sci. 2025. PMID: 40019707 Review.
-
Design and synthesis of polymer-functionalized NIR fluorescent dyes--magnetic nanoparticles for bioimaging.ACS Nano. 2013 Aug 27;7(8):6796-805. doi: 10.1021/nn401734t. Epub 2013 Jul 25. ACS Nano. 2013. PMID: 23869722
-
Recent Progress in NIR-II Contrast Agent for Biological Imaging.Front Bioeng Biotechnol. 2020 Jan 30;7:487. doi: 10.3389/fbioe.2019.00487. eCollection 2019. Front Bioeng Biotechnol. 2020. PMID: 32083067 Free PMC article. Review.
-
A General Strategy for Development of Near-Infrared Fluorescent Probes for Bioimaging.Angew Chem Int Ed Engl. 2017 Dec 22;56(52):16611-16615. doi: 10.1002/anie.201710688. Epub 2017 Nov 30. Angew Chem Int Ed Engl. 2017. PMID: 29134784 Free PMC article.
Cited by
-
Ru(II)-Catalyzed Regiospecific C-H/O-H Oxidative Annulation to Access Isochromeno[8,1-ab]phenazines: Far-Red Fluorescence and Live Cancer Cell Imaging.ACS Omega. 2017 Jun 30;2(6):2694-2705. doi: 10.1021/acsomega.7b00335. Epub 2017 Jun 17. ACS Omega. 2017. PMID: 30023674 Free PMC article.
-
A Novel Biscarbazole-Xanthene Hybrid Fluorescent Probe for Selective and Sensitive Detection of Cu2+ and Applications in Bioimaging.J Fluoresc. 2019 May;29(3):727-735. doi: 10.1007/s10895-019-02393-1. Epub 2019 May 19. J Fluoresc. 2019. PMID: 31104269
-
Triple-FRET multi-purpose fluorescent probe for three-protease detection.RSC Adv. 2022 Oct 10;12(44):28780-28787. doi: 10.1039/d2ra05125g. eCollection 2022 Oct 4. RSC Adv. 2022. PMID: 36320525 Free PMC article.
-
THQ-Xanthene: An Emerging Strategy to Create Next-Generation NIR-I/II Fluorophores.Adv Sci (Weinh). 2023 Jun;10(18):e2301177. doi: 10.1002/advs.202301177. Epub 2023 Apr 28. Adv Sci (Weinh). 2023. PMID: 37114796 Free PMC article. Review.
-
Fluorescent probes with high pKa values based on traditional, near-infrared rhodamine, and hemicyanine fluorophores for sensitive detection of lysosomal pH variations.Methods. 2019 Sep 15;168:40-50. doi: 10.1016/j.ymeth.2019.07.012. Epub 2019 Jul 22. Methods. 2019. PMID: 31344405 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

