A bilayered nanoshell for durable protection of single yeast cells against multiple, simultaneous hostile stimuli
- PMID: 29910923
- PMCID: PMC5982223
- DOI: 10.1039/c8sc01130c
A bilayered nanoshell for durable protection of single yeast cells against multiple, simultaneous hostile stimuli
Abstract
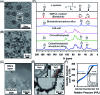
Single cell surface engineering provides the most efficient, non-genetic strategy to enhance cell stability. However, it remains a huge challenge to improve cell stability in complex artificial environments. Here, a soft biohybrid interfacial layer is fabricated on individual living-cell surfaces by their exposure to a suspension of gold nanoparticles and l-cysteine to form a protecting functional layer to which porous silica layers were bound yielding pores with a diameter of 3.9 nm. The living cells within the bilayered nanoshells maintained high viability (96 ± 2%) as demonstrated by agar plating, even after five cycles of simultaneous exposure to high temperature (40 °C), lyticase and UV light. Moreover, yeast cells encapsulated in bilayered nanoshells were more recyclable than native cells due to nutrient storage in the shell.
Figures




References
-
- Hepworth L. J., France S. P., Hussain S., Both P., Turner N. J., Flitsch S. L. ACS Catal. 2017;7:2920.
-
- Takagi T., Yokoi T., Shibata T., Morisaka H., Kuroda K., Ueda M. Appl. Microbiol. Biotechnol. 2016;100:1723. - PubMed
-
- Liu Q., Wu C., Cai H., Hu N., Zhou J., Wang P. Chem. Rev. 2014;114:6423. - PubMed
-
- Loqué D., Scheller H. V., Pauly M. Curr. Opin. Plant Biol. 2015;25:151. - PubMed
-
- Wang L., Hu Z.-Y., Yang X.-Y., Zhang B.-B., Geng W., Van Tendeloo G., Su B.-L. Chem. Commun. 2017;53:6617. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

