Engineering thermoresponsive phase separated vesicles formed via emulsion phase transfer as a content-release platform
- PMID: 29910937
- PMCID: PMC5982195
- DOI: 10.1039/c7sc04309k
Engineering thermoresponsive phase separated vesicles formed via emulsion phase transfer as a content-release platform
Abstract
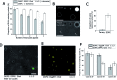
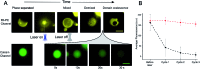
Giant unilamellar vesicles (GUVs) are a well-established tool for the study of membrane biophysics and are increasingly used as artificial cell models and functional units in biotechnology. This trend is driven by the development of emulsion-based generation methods such as Emulsion Phase Transfer (EPT), which facilitates the encapsulation of almost any water-soluble compounds (including biomolecules) regardless of size or charge, is compatible with droplet microfluidics, and allows GUVs with asymmetric bilayers to be assembled. However, the ability to control the composition of membranes formed via EPT remains an open question; this is key as composition gives rise to an array of biophysical phenomena which can be used to add functionality to membranes. Here, we evaluate the use of GUVs constructed via this method as a platform for phase behaviour studies and take advantage of composition-dependent features to engineer thermally-responsive GUVs. For the first time, we generate ternary GUVs (DOPC/DPPC/cholesterol) using EPT, and by compensating for the lower cholesterol incorporation efficiencies, show that these possess the full range of phase behaviour displayed by electroformed GUVs. As a demonstration of the fine control afforded by this approach, we demonstrate release of dye and peptide cargo when ternary GUVs are heated through the immiscibility transition temperature, and show that release temperature can be tuned by changing vesicle composition. We show that GUVs can be individually addressed and release triggered using a laser beam. Our findings validate EPT as a suitable method for generating phase separated vesicles and provide a valuable proof-of-concept for engineering content release functionality into individually addressable vesicles, which could have a host of applications in the development of smart synthetic biosystems.
Figures

Similar articles
-
Optimization of the Inverted Emulsion Method for High-Yield Production of Biomimetic Giant Unilamellar Vesicles.Chembiochem. 2019 Oct 15;20(20):2674-2682. doi: 10.1002/cbic.201900529. Epub 2019 Oct 11. Chembiochem. 2019. PMID: 31529570 Free PMC article.
-
Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine.Langmuir. 2004 Oct 26;20(22):9526-34. doi: 10.1021/la049481g. Langmuir. 2004. PMID: 15491182
-
Vesicle fission of giant unilamellar vesicles of liquid-ordered-phase membranes induced by amphiphiles with a single long hydrocarbon chain.Langmuir. 2007 Jan 16;23(2):720-8. doi: 10.1021/la062078k. Langmuir. 2007. PMID: 17209626
-
Exploring the raft-hypothesis by probing planar bilayer patches of free-standing giant vesicles at nanoscale resolution, with and without Na,K-ATPase.Biochim Biophys Acta. 2016 Dec;1858(12):3041-3049. doi: 10.1016/j.bbamem.2016.09.001. Epub 2016 Sep 9. Biochim Biophys Acta. 2016. PMID: 27616046 Review.
-
A Practical Guide to Preparation and Applications of Giant Unilamellar Vesicles Formed via Centrifugation of Water-in-Oil Emulsion Droplets.Membranes (Basel). 2023 Apr 18;13(4):440. doi: 10.3390/membranes13040440. Membranes (Basel). 2023. PMID: 37103867 Free PMC article. Review.
Cited by
-
Manufacture of Multilayered Artificial Cell Membranes through Sequential Bilayer Deposition on Emulsion Templates.Chembiochem. 2021 Jul 1;22(13):2275-2281. doi: 10.1002/cbic.202100072. Epub 2021 Mar 31. Chembiochem. 2021. PMID: 33617681 Free PMC article.
-
Giant Vesicles Produced with Phosphatidylcholines (PCs) and Phosphatidylethanolamines (PEs) by Water-in-Oil Inverted Emulsions.Life (Basel). 2021 Mar 10;11(3):223. doi: 10.3390/life11030223. Life (Basel). 2021. PMID: 33801936 Free PMC article.
-
Several common methods of making vesicles (except an emulsion method) capture intended lipid ratios.Biophys J. 2024 Oct 1;123(19):3452-3462. doi: 10.1016/j.bpj.2024.08.019. Epub 2024 Aug 26. Biophys J. 2024. PMID: 39192580
-
Fusing Artificial Cell Compartments and Lipid Domains Using Optical Traps: A Tool to Modulate Membrane Composition and Phase Behaviour.Micromachines (Basel). 2020 Apr 7;11(4):388. doi: 10.3390/mi11040388. Micromachines (Basel). 2020. PMID: 32272670 Free PMC article.
-
A Modular, Dynamic, DNA-Based Platform for Regulating Cargo Distribution and Transport between Lipid Domains.Nano Lett. 2021 Apr 14;21(7):2800-2808. doi: 10.1021/acs.nanolett.0c04867. Epub 2021 Mar 18. Nano Lett. 2021. PMID: 33733783 Free PMC article.
References
-
- Walde P., Cosentino K., Engel H., Stano P. ChemBioChem. 2010;11:848–865. - PubMed
-
- Noireaux V., Bar-Ziv R., Godefroy J., Salman H., Libchaber A. Phys. Biol. 2005;2:P1. - PubMed
-
- Luisi P. L., Ferri F., Stano P. Naturwissenschaften. 2006;93:1–13. - PubMed
-
- Thomas J. M., Friddin M. S., Ces O., Elani Y. Chem. Commun. 2017;53:12282–12285. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

