Multiple roles of aryloxide leaving groups in enantioselective annulations employing α,β-unsaturated acyl ammonium catalysis
- PMID: 29910944
- PMCID: PMC5982221
- DOI: 10.1039/c8sc01324a
Multiple roles of aryloxide leaving groups in enantioselective annulations employing α,β-unsaturated acyl ammonium catalysis
Abstract
An isothiourea-catalysed Michael addition-annulation process using β-fluoroalkyl-substituted α,β-unsaturated aryl esters and a range of 2-acylbenzazoles is reported for the enantioselective synthesis of dihydropyranone and dihydropyridinone products bearing polyfluorinated stereocenters (29 examples, up to 98% yield, >99 : 1 er). The choice of aryl group of the aryl ester proved essential in determining reaction enantioselectivity and dihydropyranone : dihydropyridinone product selectivity. The aryloxide leaving group is shown to play a number of essential additional roles, operating (i) as a Brønsted base, circumventing the need for an auxiliary base; and (ii) as a Lewis base to catalyse the isomerisation of dihydropyranone products into thermodynamically-favoured dihydropyridinones. After optimisation, this isomerisation process was exploited for the selective synthesis of dihydropyridinone products using acylbenzothiazoles, and either dihydropyranone or dihydropyridinone products using acylbenzoxazoles. Finally, the phenol derivative, produced following protonation of the aryloxide, is proposed to act as a Brønsted acid, which promotes an isothiourea-catalysed kinetic resolution of benzoxazole-derived dihydropyranones.
Figures
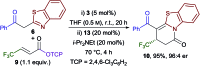

Similar articles
-
Enantioselective Synthesis of 3,5,6-Substituted Dihydropyranones and Dihydropyridinones using Isothiourea-Mediated Catalysis.Chem Asian J. 2016 Feb 4;11(3):395-400. doi: 10.1002/asia.201500907. Epub 2015 Nov 12. Chem Asian J. 2016. PMID: 26471245 Free PMC article.
-
Aryloxide-Facilitated Catalyst Turnover in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis.Angew Chem Int Ed Engl. 2017 Sep 25;56(40):12282-12287. doi: 10.1002/anie.201706402. Epub 2017 Aug 25. Angew Chem Int Ed Engl. 2017. PMID: 28791763 Free PMC article.
-
Base-free Enantioselective C(1)-Ammonium Enolate Catalysis Exploiting Aryloxides: A Synthetic and Mechanistic Study.Angew Chem Int Ed Engl. 2019 Oct 14;58(42):15111-15119. doi: 10.1002/anie.201908627. Epub 2019 Sep 12. Angew Chem Int Ed Engl. 2019. PMID: 31436380 Review.
-
Enantioselective Synthesis of α-Aryl-β2 -Amino-Esters by Cooperative Isothiourea and Brønsted Acid Catalysis.Angew Chem Int Ed Engl. 2021 May 17;60(21):11892-11900. doi: 10.1002/anie.202016220. Epub 2021 May 4. Angew Chem Int Ed Engl. 2021. PMID: 33646631 Free PMC article.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Cooperative Palladium/Isothiourea Catalyzed Enantioselective Formal (3+2) Cycloaddition of Vinylcyclopropanes and α,β-Unsaturated Esters.Angew Chem Int Ed Engl. 2022 Jun 20;61(25):e202202621. doi: 10.1002/anie.202202621. Epub 2022 Apr 28. Angew Chem Int Ed Engl. 2022. PMID: 35389553 Free PMC article.
-
Isothiourea-catalysed enantioselective Michael addition of N-heterocyclic pronucleophiles to α,β-unsaturated aryl esters.Chem Sci. 2019 Oct 23;11(1):241-247. doi: 10.1039/c9sc04303a. Chem Sci. 2019. PMID: 34040717 Free PMC article.
-
Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters.Nat Chem. 2023 May;15(5):714-721. doi: 10.1038/s41557-023-01165-6. Epub 2023 May 1. Nat Chem. 2023. PMID: 37127757 Free PMC article.
-
Understanding divergent substrate stereoselectivity in the isothiourea-catalysed conjugate addition of cyclic α-substituted β-ketoesters to α,β-unsaturated aryl esters.Chem Sci. 2023 Nov 21;14(48):14146-14156. doi: 10.1039/d3sc05470e. eCollection 2023 Dec 13. Chem Sci. 2023. PMID: 38098722 Free PMC article.
-
Carbene-catalyzed enantioselective oxidative coupling of enals and di(hetero)arylmethanes.Chem Sci. 2018 Sep 18;9(46):8711-8715. doi: 10.1039/c8sc03480j. eCollection 2018 Dec 14. Chem Sci. 2018. PMID: 30595836 Free PMC article.
References
-
- Hao L., Du Y., Lv H., Chen X., Jiang H., Shao Y., Chi Y. R. Org. Lett. 2012;14:2154–2157. - PubMed
-
-
For a review see ref. 2b. For selected examples see:
- Fu Z., Xu J., Zhu T., Leong W. W. Y., Chi Y. R. Nat. Chem. 2013;5:835–839. - PubMed
- Xu J., Jin Z., Chi Y. R. Org. Lett. 2013;15:5028–5031. - PubMed
- Cheng J., Huang Z., Chi Y. R. Angew. Chem., Int. Ed. 2013;52:8592–8596. - PubMed
- Fu Z., Wu X., Chi Y. R. Org. Chem. Front. 2016;3:145–149.
- Wang H., Chen X., Li Y., Wang J., Wu S., Xue W., Yang S., Chi Y. R. Org. Lett. 2018;20:333–336. - PubMed
-
-
- She reported that an N-hydroxyphthalimide leaving group could be exploited in a subsequent N- to C-sulfonyl transfer of N-tosyl dihydropyridinone products: Han R., He L., Liu L., Xie X., She X., Chem.–Asian J., 2016, 11 , 193 –197 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

