Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway
- PMID: 29911071
- PMCID: PMC5992419
- DOI: 10.3389/fonc.2018.00192
Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway
Abstract
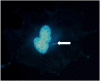
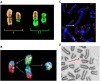
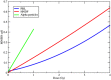
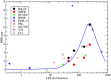
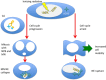
Radiation-induced chromosomal aberrations represent an early marker of late effects, including cell killing and transformation. The measurement of cytogenetic damage in tissues, generally in blood lymphocytes, from patients treated with radiotherapy has been studied for many years to predict individual sensitivity and late morbidity. Acentric fragments are lost during mitosis and create micronuclei (MN), which are well correlated to cell killing. Immunotherapy is rapidly becoming a most promising new strategy for metastatic tumors, and combination with radiotherapy is explored in several pre-clinical studies and clinical trials. Recent evidence has shown that the presence of cytosolic DNA activates immune response via the cyclic GMP-AMP synthase/stimulator of interferon genes pathway, which induces type I interferon transcription. Cytosolic DNA can be found after exposure to ionizing radiation either as MN or as small fragments leaking through nuclear envelope ruptures. The study of the dependence of cytosolic DNA and MN on dose and radiation quality can guide the optimal combination of radiotherapy and immunotherapy. The role of densely ionizing charged particles is under active investigation to define their impact on the activation of the interferon pathway.
Keywords: chromosome aberrations; cyclic GMP–AMP synthase; micronuclei; particle therapy; radioimmunotherapy; stimulator of interferon genes.
Figures

References
-
- Lea DE. Action of Radiations on Living Cells. New York, NY: Cambridge University Press, The Macmillan Company; (1947).
-
- Durante M. Radiation cytogenetics: the color revolution. In: Cigna AA, Durante M, editors. Radiation Risk Estimates in Normal and Emergency Situations NATO Security through Science Series. Dordrecht, Netherlands: Springer; (2016). p. 243–52.
-
- International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. Vienna: International Atomic Energy Agency; (2011).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

