Targeting Tumor-Associated Exosomes with Integrin-Binding Peptides
- PMID: 29911169
- PMCID: PMC6001286
- DOI: 10.1002/adbi.201600038
Targeting Tumor-Associated Exosomes with Integrin-Binding Peptides
Abstract
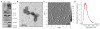
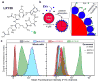
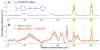
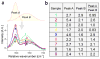
All cells expel a variety of nano-sized extracellular vesicles (EVs), including exosomes, with composition reflecting the cells' biological state. Cancer pathology is dramatically mediated by EV trafficking via key proteins, lipids, metabolites, and microRNAs. Recent proteomics evidence suggests that tumor-associated exosomes exhibit distinct expression of certain membrane proteins, rendering those proteins as attractive targets for diagnostic or therapeutic application. Yet, it is not currently feasible to distinguish circulating EVs in complex biofluids according to their tissue of origin or state of disease. Here we demonstrate peptide binding to tumor-associated EVs via overexpressed membrane protein. We find that SKOV-3 ovarian tumor cells and their released EVs express α3β1 integrin, which can be targeted by our in-house cyclic nonapeptide, LXY30. After measuring bulk SKOV-3 EV association with LXY30 by flow cytometry, Raman spectral analysis of laser-trapped single exosomes with LXY30-dialkyne conjugate enabled us to differentiate cancer-associated exosomes from non-cancer exosomes. Furthermore, we introduce the foundation for a highly specific detection platform for tumor-EVs in solution with biosensor surface-immobilized LXY30. LXY30 not only exhibits high specificity and affinity to α3β1 integrin-expressing EVs, but also reduces EV uptake into SKOV-3 parent cells, demonstrating the possibility for therapeutic application.
Keywords: biosensor; cancer; diagnostics; exosomes; optical tweezers.
Figures


References
-
- Society AC. Cancer Facts & Figures. 2015
-
- Gerçel-Taylor C, Atay S, Tullis RH, Kesimer M, Taylor DD. Analytical Biochemistry. 2012;428:44. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

