De-palmitoylation by N-(tert-Butyl) hydroxylamine inhibits AMPAR-mediated synaptic transmission via affecting receptor distribution in postsynaptic densities
- PMID: 29911316
- PMCID: PMC6488889
- DOI: 10.1111/cns.12996
De-palmitoylation by N-(tert-Butyl) hydroxylamine inhibits AMPAR-mediated synaptic transmission via affecting receptor distribution in postsynaptic densities
Abstract
Aims: Palmitoylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) subunits or their "scaffold" proteins produce opposite effects on AMPAR surface delivery. Considering AMPARs have long been identified as suitable drug targets for central nervous system (CNS) disorders, targeting palmitoylation signaling to regulate AMPAR function emerges as a novel therapeutic strategy. However, until now, much less is known about the effect of palmitoylation-deficient state on AMPAR function. Herein, we set out to determine the effect of global de-palmitoylation on AMPAR surface expression and its function, using a special chemical tool, N-(tert-Butyl) hydroxylamine (NtBuHA).
Methods: BS3 protein cross-linking, Western blot, immunoprecipitation, patch clamp, and biotin switch assay.
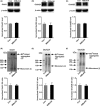
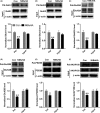
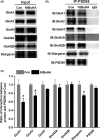
Results: Bath application of NtBuHA (1.0 mM) reduced global palmitoylated proteins in the hippocampus of mice. Although NtBuHA (1.0 mM) did not affect the expression of ionotropic glutamate receptor subunits, it preferentially decreased the surface expression of AMPARs, not N-methyl-d-aspartate receptors (NMDARs). Notably, NtBuHA (1.0 mM) reduces AMPAR-mediated excitatory postsynaptic currents (mEPSCs) in the hippocampus. This effect may be largely due to the de-palmitoylation of postsynaptic density protein 95 (PSD95) and protein kinase A-anchoring proteins, both of which stabilized AMPAR synaptic delivery. Furthermore, we found that changing PSD95 palmitoylation by NtBuHA altered the association of PSD95 with stargazin, which interacted directly with AMPARs, but not NMDARs.
Conclusion: Our data suggest that the palmitoylation-deficient state initiated by NtBuHA preferentially reduces AMPAR function, which may potentially be used for the treatment of CNS disorders, especially infantile neuronal ceroid lipofuscinosis (Batten disease).
Keywords: AMPA receptor; N-(tert-Butyl) hydroxylamine; palmitoylation; postsynaptic density protein 95; stargazin.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
All the authors report no biomedical financial interests or potential conflict of interests.
Figures



References
-
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103‐126. - PubMed
-
- van der Sluijs P, Hoogenraad CC. New insights in endosomal dynamics and AMPA receptor trafficking. Semin Cell Dev Biol. 2011;22:499‐505. - PubMed
-
- Ehlers MD. Dendritic trafficking for neuronal growth and plasticity. Biochem Soc Trans. 2013;41:1365‐1382. - PubMed
-
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161‐175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

