Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats
- PMID: 29911322
- PMCID: PMC6182564
- DOI: 10.1002/mbo3.677
Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats
Abstract
Interests in the impact of the gastrointestinal microbiota on health and wellbeing have extended from humans to that of companion animals. While relatively fewer studies to date have examined canine and feline gut microbiomes, analysis of the metagenomic DNA from fecal communities using next-generation sequencing technologies have provided insights into the microbes that are present, their function, and potential to contribute to overall host nutrition and health. As carnivores, healthy dogs and cats possess fecal microbiomes that reflect the generally higher concentrations of protein and fat in their diets, relative to omnivores and herbivores. The phyla Firmicutes and Bacteroidetes are highly abundant, and Fusobacteria, Actinobacteria, and Proteobacteria also feature prominently. Proteobacteria is the most diverse bacterial phylum and commonly features in the fecal microbiota of healthy dogs and cats, although its reputation is often sullied as its members include a number of well-known opportunistic pathogens, such as Escherichia coli, Salmonella, and Campylobacter, which may impact the health of the host and its owner. Furthermore, in other host species, high abundances of Proteobacteria have been associated with dysbiosis in hosts with metabolic or inflammatory disorders. In this review, we seek to gain further insight into the prevalence and roles of the Proteobacteria within the gastrointestinal microbiomes of healthy dogs and cats. We draw upon the growing number of metagenomic DNA sequence-based studies which now allow us take a culture-independent approach to examine the functions that this more minor, yet important, group contribute to normal microbiome function.
Keywords: Proteobacteria; 16S rRNA gene; canine; fecal microbiome; feline; metagenome.
© 2018 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Figures

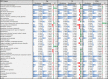
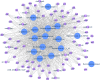
References
-
- AAFCO . (2016). Association of American Feed Control Officials 2016 Official Publication.
-
- AlShawaqfeh, M. K. , Wajid, B. , Minamoto, Y. , Markel, M. , Lidbury, J. A. , Steiner, J. M. , … Suchodolski, J. S . 2017. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiology Ecology, 93: fix136 10.1093/femsec/fix136. - DOI - PubMed
-
- Axelsson, E. , Ratnakumar, A. , Arendt, M. L. , Maqbool, K. , Webster, M. T. , Perloski, M. , … Lindblad‐Toh, K. (2013). The genomic signature of dog domestication reveals adaptation to a starch‐rich diet. Nature, 495, 364. - PubMed
-
- Baede, V. O. , Wagenaar, J. A. , Broens, E. M. , Duim, B. , Dohmen, W. , Nijsse, R. , … Hordijk, J. (2015). Longitudinal study of extended‐spectrum‐beta‐lactamase‐ and AmpC‐producing Enterobacteriaceae in household dogs. Antimicrobial Agents and Chemotherapy, 59, 3117–3124. 10.1128/AAC.04576-14 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

