Programmable Multivalent DNA-Origami Tension Probes for Reporting Cellular Traction Forces
- PMID: 29911385
- PMCID: PMC6087633
- DOI: 10.1021/acs.nanolett.8b01374
Programmable Multivalent DNA-Origami Tension Probes for Reporting Cellular Traction Forces
Abstract
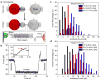
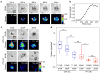
Mechanical forces are central to most, if not all, biological processes, including cell development, immune recognition, and metastasis. Because the cellular machinery mediating mechano-sensing and force generation is dependent on the nanoscale organization and geometry of protein assemblies, a current need in the field is the development of force-sensing probes that can be customized at the nanometer-length scale. In this work, we describe a DNA origami tension sensor that maps the piconewton (pN) forces generated by living cells. As a proof-of-concept, we engineered a novel library of six-helix-bundle DNA-origami tension probes (DOTPs) with a tailorable number of tension-reporting hairpins (each with their own tunable tension response threshold) and a tunable number of cell-receptor ligands. We used single-molecule force spectroscopy to determine the probes' tension response thresholds and used computational modeling to show that hairpin unfolding is semi-cooperative and orientation-dependent. Finally, we use our DOTP library to map the forces applied by human blood platelets during initial adhesion and activation. We find that the total tension signal exhibited by platelets on DOTP-functionalized surfaces increases with the number of ligands per DOTP, likely due to increased total ligand density, and decreases exponentially with the DOTP's force-response threshold. This work opens the door to applications for understanding and regulating biophysical processes involving cooperativity and multivalency.
Keywords: DNA origami; biomembrane force probe; cellular traction forces; platelets.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Evans EA, Calderwood DA. Science. 2007;316(5828):1148–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

