Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities
- PMID: 29912148
- PMCID: PMC6025361
- DOI: 10.3390/cancers10060207
Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities
Abstract
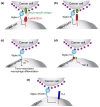
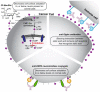
Cell surface glycosylation is dynamic and often changes in response to cellular differentiation under physiological or pathophysiological conditions. Altered glycosylation on cancers cells is gaining attention due its wide-spread occurrence across a variety of cancer types and recent studies that have documented functional roles for aberrant glycosylation in driving cancer progression at various stages. One change in glycosylation that can correlate with cancer stage and disease prognosis is hypersialylation. Increased levels of sialic acid are pervasive in cancer and a growing body of evidence demonstrates how hypersialylation is advantageous to cancer cells, particularly from the perspective of modulating immune cell responses. Sialic acid-binding receptors, such as Siglecs and Selectins, are well-positioned to be exploited by cancer hypersialylation. Evidence is also mounting that Siglecs modulate key immune cell types in the tumor microenvironment, particularly those responsible for maintaining the appropriate inflammatory environment. From these studies have come new and innovative ways to block the effects of hypersialylation by directly reducing sialic acid on cancer cells or blocking interactions between sialic acid and Siglecs or Selectins. Here we review recent works examining how cancer cells become hypersialylated, how hypersialylation benefits cancer cells and tumors, and proposed therapies to abrogate hypersialylation of cancer.
Keywords: Selectin; Siglec; glycosylation; immunosurveillance; inflammation; lectin; sialic acid; tumor-associated macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Sialic acids in cancer biology and immunity.Glycobiology. 2016 Feb;26(2):111-28. doi: 10.1093/glycob/cwv097. Epub 2015 Oct 30. Glycobiology. 2016. PMID: 26518624 Review.
-
Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer.Glycobiology. 2018 Sep 1;28(9):640-647. doi: 10.1093/glycob/cwx108. Glycobiology. 2018. PMID: 29309569 Review.
-
Aberrant Sialylation in Cancer: Therapeutic Opportunities.Cancers (Basel). 2022 Aug 31;14(17):4248. doi: 10.3390/cancers14174248. Cancers (Basel). 2022. PMID: 36077781 Free PMC article. Review.
-
A Cartography of Siglecs and Sialyltransferases in Gynecologic Malignancies: Is There a Road Towards a Sweet Future?Front Oncol. 2018 Mar 13;8:68. doi: 10.3389/fonc.2018.00068. eCollection 2018. Front Oncol. 2018. PMID: 29594046 Free PMC article.
-
The Distinct Roles of Sialyltransferases in Cancer Biology and Onco-Immunology.Front Immunol. 2021 Dec 17;12:799861. doi: 10.3389/fimmu.2021.799861. eCollection 2021. Front Immunol. 2021. PMID: 34975914 Free PMC article. Review.
Cited by
-
Development of Siglec-9 Blocking Antibody to Enhance Anti-Tumor Immunity.Front Oncol. 2021 Nov 19;11:778989. doi: 10.3389/fonc.2021.778989. eCollection 2021. Front Oncol. 2021. PMID: 34869028 Free PMC article.
-
An efficient and robust HPLC method to determine the sialylation levels of human epithelial cells.PLoS One. 2022 Jan 18;17(1):e0257178. doi: 10.1371/journal.pone.0257178. eCollection 2022. PLoS One. 2022. PMID: 35041670 Free PMC article.
-
Redox-Controlled Site-Specific α2-6-Sialylation.J Am Chem Soc. 2019 Mar 20;141(11):4547-4552. doi: 10.1021/jacs.9b00044. Epub 2019 Mar 11. J Am Chem Soc. 2019. PMID: 30843692 Free PMC article.
-
ST6GAL1 and α2-6 Sialylation Regulates IL-6 Expression and Secretion in Chronic Obstructive Pulmonary Disease.Front Immunol. 2021 Jul 5;12:693149. doi: 10.3389/fimmu.2021.693149. eCollection 2021. Front Immunol. 2021. PMID: 34290711 Free PMC article.
-
Current Status on Therapeutic Molecules Targeting Siglec Receptors.Cells. 2020 Dec 15;9(12):2691. doi: 10.3390/cells9122691. Cells. 2020. PMID: 33333862 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

