Pneumococcal Meningitis in Adults after Introduction of PCV7 and PCV13, Israel, July 2009-June 20151
- PMID: 29912694
- PMCID: PMC6038733
- DOI: 10.3201/eid2407.170721
Pneumococcal Meningitis in Adults after Introduction of PCV7 and PCV13, Israel, July 2009-June 20151
Abstract
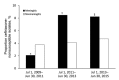
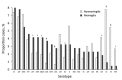
The indirect effect of pneumococcal conjugate vaccine on adult pneumococcal meningitis has not been thoroughly investigated. We present data from active surveillance on pneumococcal meningitis in adults in Israel occurring during July 2009-June 2015. Pneumococcal meningitis was diagnosed for 221 patients, 9.4% of all invasive pneumococcal disease (IPD) cases. Although overall IPD incidence decreased during the study period, meningitis increased nonsignificantly from 0.66 to 0.85 cases/100,000 population. Incidence of vaccine type (VT) pneumococcal meningitis (VT13) decreased by 70%, but non-VT13 pneumococcal meningitis increased from 0.32 to 0.75 cases/100,000 population (incident rate ratio 2.35, 95% CI 1.27-4.35). Pneumococcal meningitis patients were younger and healthier than nonmeningitis IPD patients, and 20.2% had a history of previous head surgery or cerebrospinal fluid leak compared with <2.0% of nonmeningitis patients (p<0.0001). Non-VT13 types that rarely cause IPD (15B/C, 6C, 23A, 23B, 24F) seem to be emerging as common causes of meningitis.
Keywords: Israel; PCV13; PCV7; S. pneumoniae; Streptococcus pneumoniae; adults; bacteria; indirect effects; invasive pneumococcal disease; meningitis; nationwide surveillance; pneumococcal conjugate vaccine; pneumococcal meningitis; serotype replacement; streptococci; vaccine.
Figures



References
-
- Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, et al.; IAIPD group. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: A nationwide surveillance study. Vaccine. 2015;33:1135–42. 10.1016/j.vaccine.2015.01.030 - DOI - PubMed
-
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–43. 10.1016/S1473-3099(15)70044-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

