Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk
- PMID: 29912879
- PMCID: PMC6005533
- DOI: 10.1371/journal.pone.0197464
Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk
Abstract
Background: N-glycolylneuraminic acid (Neu5Gc) is a non-human red-meat-derived sialic acid immunogenic to humans. Neu5Gc can be metabolically incorporated into glycan chains on human endothelial and epithelial surfaces. This represents the first example of a "xeno-autoantigen", against which circulating human "xeno-autoantibodies" can react. The resulting inflammation ("xenosialitis") has been demonstrated in human-like Neu5Gc-deficient mice and contributed to carcinoma progression via antibody-mediated inflammation. Anti-Neu5Gc antibodies have potential as biomarkers for diseases associated with red meat consumption such as carcinomas, atherosclerosis, and type 2 diabetes.
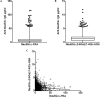
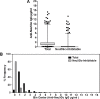
Methods: ELISA assays measured antibodies against Neu5Gc or Neu5Gc-glycans in plasma or serum samples from the Nurses' Health Studies, the Health Professionals Follow-up Study, and the European Prospective Investigation into Cancer and Nutrition, including inter-assay reproducibility, stability with delayed sample processing, and within-person reproducibility over 1-3 years in archived samples. We also assessed associations between antibody levels and coronary artery disease risk (CAD) or red meat intake. A glycan microarray was used to detected antibodies against multiple Neu5Gc-glycan epitopes. A nested case-control study design assessed the association between total anti-Neu5Gc antibodies detected in the glycan array assay and the risk of colorectal cancer (CRC).
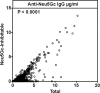
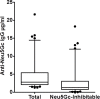
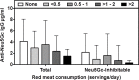
Results: ELISA assays showed a wide range of anti-Neu5Gc responses and good inter-assay reproducibility, stability with delayed sample processing, and within-person reproducibility over time, but these antibody levels did not correlate with CAD risk or red meat intake. Antibodies against Neu5Gc alone or against individual Neu5Gc-bearing epitopes were also not associated with colorectal cancer (CRC) risk. However, a sialoglycan microarray study demonstrated positive association with CRC risk when the total antibody responses against all Neu5Gc-glycans were combined. Individuals in the top quartile of total anti-Neu5Gc IgG antibody concentrations had nearly three times the risk compared to those in the bottom quartile (Multivariate Odds Ratio comparing top to bottom quartile: 2.98, 95% CI: 0.80, 11.1; P for trend = 0.02).
Conclusions: Further work harnessing the utility of these anti-Neu5Gc antibodies as biomarkers in red meat-associated diseases must consider diversity in individual antibody profiles against different Neu5Gc-bearing glycans. Traditional ELISA assays for antibodies directed against Neu5Gc alone, or against specific Neu5Gc-glycans may not be adequate to define risk associations. Our finding of a positive association of total anti-Neu5Gc antibodies with CRC risk also warrants confirmation in larger prospective studies.
Conflict of interest statement
The authors have read the journal’s policy and have the following conflicts: Zahra Khedri is affiliated with Ajinomoto Althea, Dzung Nguyen is affiliated with BioLegend Inc., and Christopher J. Gregg is affiliated with GRO Biosciences. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study.BMC Med. 2020 Sep 23;18(1):262. doi: 10.1186/s12916-020-01721-8. BMC Med. 2020. PMID: 32962714 Free PMC article.
-
Presentation Mode of Glycans Affect Recognition of Human Serum anti-Neu5Gc IgG Antibodies.Bioconjug Chem. 2019 Jan 16;30(1):161-168. doi: 10.1021/acs.bioconjchem.8b00817. Epub 2018 Dec 13. Bioconjug Chem. 2019. PMID: 30500162 Free PMC article.
-
From "Serum Sickness" to "Xenosialitis": Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc.Front Immunol. 2019 Apr 17;10:807. doi: 10.3389/fimmu.2019.00807. eCollection 2019. Front Immunol. 2019. PMID: 31057542 Free PMC article. Review.
-
A red meat-derived glycan promotes inflammation and cancer progression.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):542-7. doi: 10.1073/pnas.1417508112. Epub 2014 Dec 29. Proc Natl Acad Sci U S A. 2015. PMID: 25548184 Free PMC article.
-
Involvement of a non-human sialic Acid in human cancer.Front Oncol. 2014 Feb 19;4:33. doi: 10.3389/fonc.2014.00033. eCollection 2014. Front Oncol. 2014. PMID: 24600589 Free PMC article. Review.
Cited by
-
Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study.BMC Med. 2020 Sep 23;18(1):262. doi: 10.1186/s12916-020-01721-8. BMC Med. 2020. PMID: 32962714 Free PMC article.
-
N-Glycolylneuraminic Acid (Neu5Gc) Null Large Animals by Targeting the CMP-Neu5Gc Hydroxylase (CMAH).Front Immunol. 2019 Oct 15;10:2396. doi: 10.3389/fimmu.2019.02396. eCollection 2019. Front Immunol. 2019. PMID: 31681287 Free PMC article. Review.
-
N-glycolylated carbohydrates in nature.Glycobiology. 2022 Oct 31;32(11):921-932. doi: 10.1093/glycob/cwac048. Glycobiology. 2022. PMID: 35925816 Free PMC article. Review.
-
Differential Recognition of Diet-Derived Neu5Gc-Neoantigens on Glycan Microarrays by Carbohydrate-Specific Pooled Human IgG and IgA Antibodies.Bioconjug Chem. 2019 May 15;30(5):1565-1574. doi: 10.1021/acs.bioconjchem.9b00273. Epub 2019 Apr 24. Bioconjug Chem. 2019. PMID: 30994337 Free PMC article.
-
Presentation Mode of Glycans Affect Recognition of Human Serum anti-Neu5Gc IgG Antibodies.Bioconjug Chem. 2019 Jan 16;30(1):161-168. doi: 10.1021/acs.bioconjchem.8b00817. Epub 2018 Dec 13. Bioconjug Chem. 2019. PMID: 30500162 Free PMC article.
References
-
- Pinho SS, Reis CA (2015) Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 15: 540–555. doi: 10.1038/nrc3982 - DOI - PubMed
-
- Varki A, Kannagi R, Toole B, Stanley P (2017) Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M et al., editors. Essentials of Glycobiology Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; doi: 10.1186/s12885-017-3891-3 - DOI
-
- Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K (2011) Cancer vaccines and carbohydrate epitopes. Vaccine 29: 8802–8826. doi: 10.1016/j.vaccine.2011.09.009 - DOI - PMC - PubMed
-
- Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U et al. (2010) Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Res 70: 1306–1313. doi: 10.1158/0008-5472.CAN-09-2893 - DOI - PMC - PubMed
-
- Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M et al. (2011) Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res 13: R25 doi: 10.1186/bcr2841 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

