The Chlamydia trachomatis PmpD adhesin forms higher order structures through disulphide-mediated covalent interactions
- PMID: 29912892
- PMCID: PMC6005502
- DOI: 10.1371/journal.pone.0198662
The Chlamydia trachomatis PmpD adhesin forms higher order structures through disulphide-mediated covalent interactions
Abstract
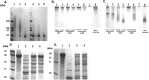
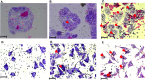
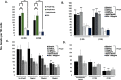
Chlamydia trachomatis (Ct) is the most common sexually transmitted bacterial pathogen, and the leading cause of infectious blindness worldwide. We have recently shown that immunization with the highly conserved antigenic passenger domain of recombinant Ct polymorphic membrane protein D (rPmpD) is protective in the mouse model of Ct genital tract infection, and previously, that ocular anti-rPmpD antibodies are elicited following vaccination. However, the mechanisms governing the assembly and structure-function relationship of PmpD are unknown. Here, we provide a biophysical analysis of this immunogenic 65 kDa passenger domain fragment of PmpD. Using differential cysteine labeling coupled with LC-MS/MS analysis, we show that widespread intra- and intermolecular disulphide interactions play important roles in the preservation of native monomeric secondary structure and the formation of higher-order oligomers. While it has been proposed that FxxN and GGA(I, L,V) repeat motifs in the Pmp21 ortholog in Chlamydia pneumoniae mediate self-interaction, no such role has previously been identified for cysteine residues in chlamydial Pmps. Further characterisation reveals that oligomeric proteoforms and rPmpD monomers adopt β-sheet folds, consistent with previously described Gram-negative bacterial type V secretion systems (T5SSs). We also highlight adhesin-like properties of rPmpD, showing that both soluble rPmpD and anti-rPmpD serum from immunized mice abrogate binding of rPmpD-coated beads to mammalian cells in a dose-dependent fashion. Hence, our study provides further evidence that chlamydial Pmps may function as adhesins, while elucidating yet another important mechanism of self-association of bacterial T5SS virulence factors that may be unique to the Chlamydiaceae.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5(2):149–61. Epub 2005/02/03. doi: 10.1038/nri1551 . - DOI - PubMed
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10(12):e0143304 Epub 2015/12/10. doi: 10.1371/journal.pone.0143304 ; PubMed Central PMCID: PMC4672879. - DOI - PMC - PubMed
-
- Peipert JF. Clinical practice. Genital chlamydial infections. N Engl J Med. 2003;349(25):2424–30. Epub 2003/12/19. doi: 10.1056/NEJMcp030542 . - DOI - PubMed
-
- Harris SR, Clarke IN, Seth-Smith HM, Solomon AW, Cutcliffe LT, Marsh P, et al. Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing. Nat Genet. 2012;44(4):413–9, S1. doi: 10.1038/ng.2214 ; PubMed Central PMCID: PMCPMC3378690. - DOI - PMC - PubMed
-
- Molleken K, Becker E, Hegemann JH. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog. 2013;9(4):e1003325 Epub 2013/05/02. doi: 10.1371/journal.ppat.1003325 ; PubMed Central PMCID: PMC3635982. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

