Enhancement of bradykinin-induced relaxation by focal brain ischemia in the rat middle cerebral artery: Receptor expression upregulation and activation of multiple pathways
- PMID: 29912902
- PMCID: PMC6005516
- DOI: 10.1371/journal.pone.0198553
Enhancement of bradykinin-induced relaxation by focal brain ischemia in the rat middle cerebral artery: Receptor expression upregulation and activation of multiple pathways
Abstract
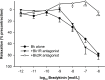
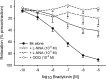
Focal brain ischemia markedly affects cerebrovascular reactivity. So far, these changes have mainly been related to alterations in the level of smooth muscle cell function while alterations of the endothelial lining have not yet been studied in detail. We have, therefore, investigated the effects of ischemia/reperfusion injury on bradykinin (BK)-induced relaxation since BK is an important mediator of tissue inflammation and affects vascular function in an endothelium-dependent manner. Focal brain ischemia was induced in rats by endovascular filament occlusion (2h) of the middle cerebral artery (MCA). After 22h reperfusion, both MCAs were harvested and the response to BK studied in organ bath experiments. Expression of the BK receptor subtypes 1 and 2 (B1, B2) was determined by real-time semi-quantitative RT-qPCR methodology, and whole mount immunofluorescence staining was performed to show the B2 receptor protein expression. In control animals, BK did not induce significant vasomotor effects despite a functionally intact endothelium and robust expression of B2 mRNA. After ischemia/reperfusion injury, BK induced a concentration-related sustained relaxation in all arteries studied, more pronounced in the ipsilateral than in the contralateral MCA. The B2 mRNA was significantly upregulated and the B1 mRNA displayed de novo expression, again more pronounced ipsi- than contralaterally. Endothelial cells displaying B2 receptor immunofluorescence were observed scattered or clustered in previously occluded MCAs. Relaxation to BK was mediated by B2 receptor activation, abolished after endothelium denudation, and largely diminished by blocking nitric oxide (NO) release or soluble guanylyl cyclase activity. Relaxation to BK was partially inhibited by charybdotoxin (ChTx), but not apamin or iberiotoxin suggesting activation of an endothelium-dependent hyperpolarization pathway. When the NO-cGMP pathway was blocked, BK induced a transient relaxation which was suppressed by ChTx. After ischemia/reperfusion injury BK elicits endothelium-dependent relaxation which was not detectable in control MCAs. This gain of function is mediated by B2 receptor activation and involves the release of NO and activation of an endothelium-dependent hyperpolarization. It goes along with increased B2 mRNA and protein expression, leaving the functional role of the de novo B1 receptor expression still open.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Stimulation of bradykinin B2-receptors on endothelial cells induces relaxation and contraction in porcine basilar artery in vitro.Br J Pharmacol. 1999 Sep;128(1):241-7. doi: 10.1038/sj.bjp.0702783. Br J Pharmacol. 1999. PMID: 10498858 Free PMC article.
-
Bradykinin dilates rat middle cerebral artery and its large branches via endothelial B2 receptors and release of nitric oxide.Peptides. 1996;17(8):1373-8. doi: 10.1016/s0196-9781(96)00223-9. Peptides. 1996. PMID: 8971934
-
Inhibition of RNA synthesis by bradykinin involves both the B1 and B2 receptor subtypes.Arch Biochem Biophys. 1996 Apr 1;328(1):115-21. doi: 10.1006/abbi.1996.0150. Arch Biochem Biophys. 1996. PMID: 8638919
-
Vasomotor and permeability effects of bradykinin in the cerebral microcirculation.Immunopharmacology. 1996 Jun;33(1-3):257-63. doi: 10.1016/0162-3109(96)00068-9. Immunopharmacology. 1996. PMID: 8856159 Review.
-
Bradykinin Receptors in Ischemic Injury.Curr Neurovasc Res. 2018;15(4):359-366. doi: 10.2174/1567202616666181123151629. Curr Neurovasc Res. 2018. PMID: 30468128 Review.
Cited by
-
Recombinant Human Tissue Kallikrein-1 for Treating Acute Ischemic Stroke and Preventing Recurrence.Stroke. 2025 Mar;56(3):745-753. doi: 10.1161/STROKEAHA.124.048858. Epub 2025 Jan 6. Stroke. 2025. PMID: 39758014 Free PMC article. Review.
-
A Phase 1C, Open Label, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of DM199 Administered Intravenously with a Polyvinyl Chloride Bag in Adult Healthy Subjects and Adults Recently Taking Angiotensin-Converting Enzyme Inhibitors.Clin Pharmacol Drug Dev. 2025 Jun;14(6):452-460. doi: 10.1002/cpdd.1534. Epub 2025 Apr 16. Clin Pharmacol Drug Dev. 2025. PMID: 40237565 Free PMC article. Clinical Trial.
References
-
- Hori S (1968) The presence of bradykinin-like polypeptides, kinin-releasing and destroying activity in brain. Jpn J Physiol 18(6):772–87. - PubMed
-
- Unterberg A, Wahl M, Baethmann A (1984) Effects of bradykinin on permeability and diameter of pial vessels in vivo. J Cereb Blood Flow Metab 4(4):574–85. doi: 10.1038/jcbfm.1984.82 - DOI - PubMed
-
- Görlach C, Hortobagyi T, Hortobagyi S, Benyo Z, Relton J, Whalley ET, et al. (2001) Bradykinin B2, but not B1, receptor antagonism has a neuroprotective effect after brain injury. J Neurotrauma 18(8):833–8. doi: 10.1089/089771501316919193 . - DOI - PubMed
-
- Plesnila N, Schulz J, Stoffel M, Eriskat J, Pruneau D, Baethmann A (2001) Role of bradykinin B2 receptors in the formation of vasogenic brain edema in rats. J Neurotrauma 18(10):1049–58. doi: 10.1089/08977150152693746 - DOI - PubMed
-
- Rachinsky M, Pruneau D, Artru AA, Kapuler V, Alonchin A, Smolanezki Y, et al. (2001) The importance of kinin antagonist treatment timing in closed head trauma. J Trauma 51(5):944–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

