Impact of delipidated estrous sheep serum supplementation on in vitro maturation, cryotolerance and endoplasmic reticulum stress gene expression of sheep oocytes
- PMID: 29912910
- PMCID: PMC6005475
- DOI: 10.1371/journal.pone.0198742
Impact of delipidated estrous sheep serum supplementation on in vitro maturation, cryotolerance and endoplasmic reticulum stress gene expression of sheep oocytes
Abstract
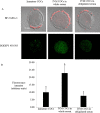
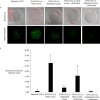
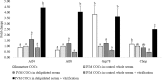
High lipid content of oocytes and embryos in domestic animals is one of the well-known factors associated with poor cryosurvival. Herein, we wanted to determine whether the use of delipidated estrous sheep serum during in vitro maturation (IVM) of ovine oocytes reduces the cytoplasmic lipid droplets content and improves embryo development and cryotolerance after vitrification. Cumulus oocytes complexes (COCs) were matured in vitro for 24 h in medium supplemented with whole or delipidated estrous sheep serum prior to vitrification. Neutral lipid present in lipid droplets of COCs, cleavage rate, embryo development rate on Day 6 and Day 8, and hatching rate on Day 8, were compared among experimental groups. Endoplasmic reticulum stress genes were evaluated in in vitro matured COCs under different lipid conditions prior to vitrification. The lipid droplets' content (mean fluorescence intensity) of oocytes cultured with IVM media supplemented with delipidated serum was lower than COCs matured with whole serum (7.6 ± 1.7 vs. 22.8 ± 5.0 arbitrary units, respectively; P< 0.05). Despite IVM treatment, oocytes subjected to vitrification showed impaired competence compared with the non-vitrified groups (P<0.05). No significant differences in embryo production were observed in non-vitrified COCs after maturation in delipidated or whole serum (33.4±4.9 vs 31.9 ±4.2). COCs matured in delipidated serum and subjected to vitrification showed increased expression of ATF4, ATF6, GRP78, and CHOP10 genes (ER stress markers). Collectively, our results demonstrate that although supplementation of IVM medium with delipidated estrous sheep serum reduces the presence of cytoplasmic lipid droplets in oocytes after maturation, oocyte cryotolerance is not improved. Notably, the expression of genes associated with the unfolded protein response (UPR) was increased in COCs, with fewer lipid droplets subjected to vitrification, suggesting that oocyte cryopreservation is associated with ER stress and activation of adaptive responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Does the Addition of Salubrinal to in vitro Maturation Medium Enhance Bovine Blastocyst Yields and Embryo Cryotolerance?Cryo Letters. 2018 May/Jun;39(3):219-226. Cryo Letters. 2018. PMID: 30059569
-
Effects of co-incubation with conspecific ampulla oviductal epithelial cells and media composition on cryotolerance and developmental competence of in vitro matured sheep oocytes.Theriogenology. 2018 Oct 15;120:10-15. doi: 10.1016/j.theriogenology.2018.07.032. Epub 2018 Jul 29. Theriogenology. 2018. PMID: 30081243
-
Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification.Mol Hum Reprod. 2016 Dec;22(12):867-881. doi: 10.1093/molehr/gaw059. Epub 2016 Sep 7. Mol Hum Reprod. 2016. PMID: 27604460
-
Impact of reducing lipid content during in vitro embryo production: A systematic review and meta-analysis.Theriogenology. 2024 Jul 1;222:31-44. doi: 10.1016/j.theriogenology.2024.04.003. Epub 2024 Apr 9. Theriogenology. 2024. PMID: 38615434
-
Endoplasmic Reticulum (ER) Stress and Unfolded Protein Response (UPR) in Mammalian Oocyte Maturation and Preimplantation Embryo Development.Int J Mol Sci. 2019 Jan 18;20(2):409. doi: 10.3390/ijms20020409. Int J Mol Sci. 2019. PMID: 30669355 Free PMC article. Review.
Cited by
-
CRISPR in livestock: From editing to printing.Theriogenology. 2020 Jul 1;150:247-254. doi: 10.1016/j.theriogenology.2020.01.063. Epub 2020 Jan 29. Theriogenology. 2020. PMID: 32088034 Free PMC article. Review.
-
Transcriptome Analysis of mRNAs and Long Non-Coding RNAs During Subsequent Embryo Development of Porcine Cloned Zygotes After Vitrification.Front Genet. 2021 Dec 17;12:753327. doi: 10.3389/fgene.2021.753327. eCollection 2021. Front Genet. 2021. PMID: 34976007 Free PMC article.
-
Lipid Signaling During Gamete Maturation.Front Cell Dev Biol. 2022 Jun 24;10:814876. doi: 10.3389/fcell.2022.814876. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36204680 Free PMC article. Review.
-
The roles of reactive oxygen species and antioxidants in cryopreservation.Biosci Rep. 2019 Aug 29;39(8):BSR20191601. doi: 10.1042/BSR20191601. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31371631 Free PMC article. Review.
-
Characterization of the Lipid Profile of Immature and In Vitro Matured Cat Oocytes.Mol Reprod Dev. 2025 Aug;92(8):e70052. doi: 10.1002/mrd.70052. Mol Reprod Dev. 2025. PMID: 40873000 Free PMC article.
References
-
- Wirtu G, Mcgill J, Crawford L, Reddy G, Bergen WG, Simon L. Targeting Lipid Metabolism to Improve Oocyte Cryopreservation (OCP) in Domestic Animals. 2013;1:15–20.
-
- Vajta G. Vitrification of the oocytes and embryos of domestic animals. Anim. Reprod. Sci. 2000;60–61:357–64. - PubMed
-
- Menchaca A, Barrera N, Santos PC dos, Cuadro F, Crispo M. Advances and limitations of in vitro embryo production in sheep and goats. Anim. Reprod. [Internet]. 2016;13:273–8. Available from: http://www.cbra.org.br/pages/publicacoes/animalreproduction/issues/downl...
-
- Wrenzycki C, Stinshoff H. Maturation environment and impact on subsequent developmental competence of bovine oocytes. Reprod. Domest. Anim. Germany; 2013;48 Suppl 1:38–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

