Efficacy of Rituximab vs Tacrolimus in Pediatric Corticosteroid-Dependent Nephrotic Syndrome: A Randomized Clinical Trial
- PMID: 29913001
- PMCID: PMC6142920
- DOI: 10.1001/jamapediatrics.2018.1323
Efficacy of Rituximab vs Tacrolimus in Pediatric Corticosteroid-Dependent Nephrotic Syndrome: A Randomized Clinical Trial
Erratum in
-
Omitted Dates of Ethical Approval and Timing of Patient Recruitment.JAMA Pediatr. 2018 Dec 1;172(12):1205. doi: 10.1001/jamapediatrics.2018.3632. JAMA Pediatr. 2018. PMID: 30304347 Free PMC article. No abstract available.
Abstract
Importance: Calcineurin inhibitors are an established first-line corticosteroid-sparing therapy for patients with corticosteroid-dependent nephrotic syndrome (CDNS), whereas B-lymphocyte-depleting therapy is mostly used as a rescue for calcineurin inhibitor-resistant cases. The positive efficacy and safety profile of rituximab raises the question of whether it could be used as a first-line alternative to calcineurin inhibitor therapy.
Objective: To compare the efficacy of rituximab and tacrolimus in maintaining relapse-free survival among children with CDNS.
Design, setting, and participants: A parallel-arm, open-label, randomized clinical trial was performed from May 8, 2015, to September 20, 2016, with 1-year follow-up in a single-center, tertiary care unit. A total of 176 consecutive children aged 3 to 16 years with CDNS not previously treated with corticosteroid-sparing agents were screened for eligibility.
Interventions: The children received either tacrolimus (along with tapering alternate-day prednisolone) for 12 months or a single course of rituximab (2 infusions of 375 mg/m2).
Main outcomes and measures: Twelve-month relapse-free survival in the intention-to-treat population.
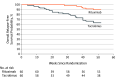
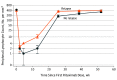
Results: Of the 176 children screened for eligibility, 120 were randomized and all but 3 patients completed 1 year of follow-up. The groups were comparable, with mean (SD) age of 7.2 (2.8) years, 32 boys (53.3%) in each group, mean (SD) disease duration of 2.5 (1.5) years and 2.3 (1.7) in the tacrolimus and rituximab groups, respectively, disease duration less than 1 year among 15 children (25.0%) in each group, median (interquartile range) of 4 (3-5) relapses in each group, and mean (SD) cumulative prednisolone dose of 246 (48) mg/kg and 239 (52) mg/kg in the prestudy year in the tacrolimus and rituximab groups, respectively. Rituximab therapy was associated with a higher 12-month relapse-free survival rate than tacrolimus (54 [90.0%] vs 38 [63.3%] children; P < .001; odds ratio, 5.21; 95% CI, 1.93-14.07). Among the patients who experienced relapse, median time to first relapse was 40 weeks in the rituximab group and 29 weeks in the tacrolimus group. Only 2 patients in the rituximab group had more than 1 relapse during the study period compared with 10 patients in the tacrolimus group. The cumulative corticosteroid dose during the 12-month study period was lower with rituximab compared with tacrolimus (mean [SD], 25.8 [27.8] vs 86.3 [58.0] mg/kg). Although both treatments were well tolerated, mild to moderate infections were twice as common in the tacrolimus group (26 [43.3%] vs 13 [21.7%] events).
Conclusions and relevance: In children with CDNS, rituximab appears to be more effective than tacrolimus in maintaining disease remission and minimizing corticosteroid exposure and, given its good tolerability and lack of nephrotoxic effects, may be considered as first-line corticosteroid-sparing therapy.
Trial registration: ClinicalTrials.gov Identifier: NCT02438982; Clinical Trial Registry of India: CTRI/2014/01/004355.
Conflict of interest statement
Figures

Comment in
-
Common Threads in Pediatric Inflammatory Diseases: Insight Into Personalized Medicine.JAMA Pediatr. 2018 Aug 1;172(8):721-722. doi: 10.1001/jamapediatrics.2018.1169. JAMA Pediatr. 2018. PMID: 29913007 Free PMC article. No abstract available.
References
-
- Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev. 2013;10(10):CD002290. - PubMed
-
- Lombel RM, Gipson DS, Hodson EM; Kidney Disease: Improving Global Outcomes . Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol. 2013;28(3):415-426. - PubMed
-
- Morgan C, Sis B, Pinsk M, Yiu V. Renal interstitial fibrosis in children treated with FK506 for nephrotic syndrome. Nephrol Dial Transplant. 2011;26(9):2860-2865. - PubMed
-
- Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583-595. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

