Effective control of neuropathic pain by transient expression of hepatocyte growth factor in a mouse chronic constriction injury model
- PMID: 29913557
- PMCID: PMC6113864
- DOI: 10.1096/fj.201800476R
Effective control of neuropathic pain by transient expression of hepatocyte growth factor in a mouse chronic constriction injury model
Abstract
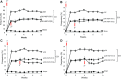
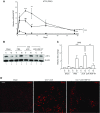
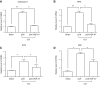
Hepatocyte growth factor (HGF) is a multifunctional protein that contains angiogenic and neurotrophic properties. In the current study, we investigated the analgesic effects of HGF by using a plasmid DNA that was designed to express 2 isoforms of human HGF-pCK-HGF-X7 (or VM202)-in a chronic constriction injury (CCI) -induced mouse neuropathic pain model. Intramuscular injection of pCK-HGF-X7 into proximal thigh muscle induced the expression of HGF in the muscle, sciatic nerve, and dorsal root ganglia (DRG). This gene transfer procedure significantly attenuated mechanical allodynia and thermal hyperalgesia after CCI. Injury-induced expression of activating transcription factor 3, calcium channel subunit α2δ1, and CSF1 in the ipsilateral DRG neurons was markedly down-regulated in the pCK-HGF-X7-treated group, which suggested that HGF might exert its analgesic effects by inhibiting pain-mediating genes in the sensory neurons. In addition, suppressed CSF1 expression in DRG neurons by pCK-HGF-X7 treatment was accompanied by a noticeable suppression of the nerve injury-induced glial cell activation in the spinal cord dorsal horn. Taken together, our data show that pCK-HGF-X7 attenuates nerve injury-induced neuropathic pain by inhibiting pain-related factors in DRG neurons and subsequent spinal cord glial activation, which suggests its therapeutic efficacy in the treatment of neuropathic pain.-Nho, B., Lee, J., Lee, J., Ko, K. R., Lee, S. J., Kim, S. Effective control of neuropathic pain by transient expression of hepatocyte growth factor in a mouse chronic constriction injury model.
Keywords: DRG; VM202; gene therapy; microglia; plasmid DNA.
Conflict of interest statement
The authors thank Subin Kim (ViroMed) for providing the pCK-HGF-X7 plasmid and Hyoungsub Lim (School of Dentistry, Seoul National University) for technical support. This work was supported by grants from ViroMed. B.N. and S.J.L. declare no conflicts of interest.
Figures






Similar articles
-
Gabapentin inhibits the analgesic effects and nerve regeneration process induced by hepatocyte growth factor (HGF) in a peripheral nerve injury model: Implication for the use of VM202 and gabapentinoids for peripheral neuropathy.Mol Cell Neurosci. 2022 Sep;122:103767. doi: 10.1016/j.mcn.2022.103767. Epub 2022 Aug 23. Mol Cell Neurosci. 2022. PMID: 36007867
-
Intramuscular injection of a plasmid DNA vector expressing hepatocyte growth factor (HGF) ameliorated pain symptoms by controlling the expression of pro-inflammatory cytokines in the dorsal root ganglion.Biochem Biophys Res Commun. 2022 Jun 4;607:60-66. doi: 10.1016/j.bbrc.2022.03.125. Epub 2022 Mar 26. Biochem Biophys Res Commun. 2022. PMID: 35366545
-
Efficacy of nonviral gene transfer of human hepatocyte growth factor (HGF) against ischemic-reperfusion nerve injury in rats.PLoS One. 2020 Aug 11;15(8):e0237156. doi: 10.1371/journal.pone.0237156. eCollection 2020. PLoS One. 2020. PMID: 32780756 Free PMC article.
-
[Contribution of primary sensory neurons and spinal glial cells to pathomechanisms of neuropathic pain].Brain Nerve. 2008 May;60(5):483-92. Brain Nerve. 2008. PMID: 18516970 Review. Japanese.
-
Role of the immune system in neuropathic pain.Scand J Pain. 2019 Dec 18;20(1):33-37. doi: 10.1515/sjpain-2019-0138. Scand J Pain. 2019. PMID: 31730538 Review.
Cited by
-
Hepatocyte growth factor is necessary for efficient outgrowth of injured peripheral axons in in vitro culture system and in vivo nerve crush mouse model.Biochem Biophys Rep. 2021 Mar 3;26:100973. doi: 10.1016/j.bbrep.2021.100973. eCollection 2021 Jul. Biochem Biophys Rep. 2021. PMID: 33718632 Free PMC article.
-
Impairment of peripheral nerve regeneration by insufficient activation of the HGF/c-Met/c-Jun pathway in aged mice.Heliyon. 2022 Nov 6;8(11):e11411. doi: 10.1016/j.heliyon.2022.e11411. eCollection 2022 Nov. Heliyon. 2022. PMID: 36387562 Free PMC article.
-
HGF and MET: From Brain Development to Neurological Disorders.Front Cell Dev Biol. 2021 Jun 9;9:683609. doi: 10.3389/fcell.2021.683609. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34179015 Free PMC article. Review.
-
Construction of Plasmid DNA Expressing Two Isoforms of Insulin-Like Growth Factor-1 and Its Effects on Skeletal Muscle Injury Models.Hum Gene Ther. 2022 Dec;33(23-24):1305-1314. doi: 10.1089/hum.2022.103. Hum Gene Ther. 2022. PMID: 35838121 Free PMC article.
-
Novel rAAV vector mediated intrathecal HGF delivery has an impact on neuroimmune modulation in the ALS motor cortex with TDP-43 pathology.Gene Ther. 2023 Aug;30(7-8):560-574. doi: 10.1038/s41434-023-00383-4. Epub 2023 Feb 24. Gene Ther. 2023. PMID: 36823441
References
-
- Gore M., Brandenburg N. A., Dukes E., Hoffman D. L., Tai K. S., Stacey B. (2005) Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain Symptom Manage. 30, 374–385 10.1016/j.jpainsymman.2005.04.009 - DOI - PubMed
-
- Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 10.1083/jcb.119.3.629 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

