Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite
- PMID: 29914092
- PMCID: PMC6024974
- DOI: 10.3390/md16060211
Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite
Abstract
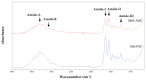
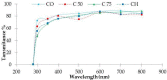
The acid solubilised collagen (ASC) and pepsin solubilised collagen (PSC) were extracted from the by-products (skin) of a cartilaginous fish (Mustelus mustelus). The ASC and PSC yields were 23.07% and 35.27% dry weight, respectively and were identified as collagen Type I with the presence of α, β and γ chains. As revealed by the Fourier Transform Infrared (FTIR) spectra analysis, pepsin did not alter the PSC triple helix structure. Based on the various type of collagen yield, only PSC was used in combination with chitosan to produce a composite film. Such film had lower tensile strength but higher elongation at break when compared to chitosan film; and lower water solubility and lightness when compared to collagen film. Equally, FTIR spectra analysis of film composite showed the occurrence of collagen-chitosan interaction resulting in a modification of the secondary structure of collagen. Collagen-chitosan-based biofilm showed a potential UV barrier properties and antioxidant activity, which might be used as green bioactive films to preserve nutraceutical products.
Keywords: cartilaginous fish by-products; chitosan; collagen; composite films; properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- FAO . Contributing to Food Security and Nutrition for All. FAO; Rome, Italy: 2016. The State of World Fisheries and Aquaculture 2016; p. 224.
-
- Birk D.E., Bruckner P. Collagen suprastructures. Collagen. 2005;247:185–205. doi: 10.1007/b103823. - DOI
-
- Kadler K. Extracellular matrix 1: Fibril-forming collagens. Protein Profile. 1995;2:491–619. - PubMed
-
- Hood L.L. Collagen in sausage casings. In: Pearson A.M., Dutson T.R., Bailey A.J., editors. Advances in Meat Research Volume 4: Collagen as Food. Volume 4. Van Nostrand Reinhold Company; New York, NY, USA: 1987. pp. 109–129.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

