Preparation of Enzyme-Activated Thapsigargin Prodrugs by Solid-Phase Synthesis
- PMID: 29914143
- PMCID: PMC6100299
- DOI: 10.3390/molecules23061463
Preparation of Enzyme-Activated Thapsigargin Prodrugs by Solid-Phase Synthesis
Abstract
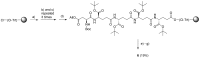
Since cells in solid tumors divide less rapidly than cells in the bone marrow or cells of the immune system, mitotic inhibitors often cause severe side effects when used for treatment of diseases like prostate cancer and breast cancer. One approach to overcome this problem involves attempts at developing drugs based on general cytotoxins, like calicheamicin and thapsigargin, which kill cells at all phases of the cell cycle. However, such toxins can only be used when efficient targeting to the malignant tissue is possible. In the case of thapsigargin, selectivity for tumor-associated cells is achieved by conjugating the drug to a peptide that is only cleaved in the vicinity of tumors to release the cytotoxic drug or an analog with retained activity. Solid-phase synthesis protocols were developed for preparation of three already validated prodrugs of thapsigargin: one prodrug cleavable by human kallikrein 2, one prodrug cleavable by prostate-specific antigen, and one prodrug cleavable by prostate-specific membrane antigen.
Keywords: mipsagargin; prodrug; solid-phase peptide synthesis: cytotoxin-peptide conjugation; targeted chemotherapy; thapsigargin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



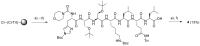
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

