Evaluation of the impact of body mass index on warfarin requirements in hospitalized patients
- PMID: 29914293
- PMCID: PMC6041876
- DOI: 10.1177/1753944718781295
Evaluation of the impact of body mass index on warfarin requirements in hospitalized patients
Abstract
Background: Despite well established empiric dose adjustments for drug and disease-state interactions, the impact of body mass index (BM) on warfarin remains unclear. The objective of this study is to evaluate warfarin requirements in hospitalized patients, stratified by BMI.
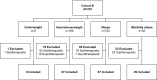
Methods: This retrospective review included two cohorts of patients: cohort A (patients admitted with a therapeutic international normalized ratio (INR)) and cohort B (newly initiated on warfarin during hospitalization). Exclusion criteria included: age under 18 years, pregnancy, INR (goal 2.5-3.5), and warfarin thromboprophylaxis post orthopedic surgery. The primary outcome was mean total weekly dose (TWD) of warfarin based on weight classification: underweight (BMI <18 kg/m2), normal/overweight (BMI 18-29.9 kg/m2), obese (BMI 30-39.9 kg/m2), and morbidly obese (BMI ⩾ 40 kg/m2). Data were extracted from two community hospitals in reverse chronologic order during July 2015-June 2013 until both study institutions evaluated 100 patients per cohort in each BMI classification or until all patients had been evaluated within the prespecified timeframe.
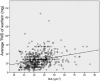
Results: A total of 585 patients were included in cohort A (26 underweight, 200 normal/overweight, 200 obese, 159 morbidly obese). There was a statistically significant difference in TWD as determined by one-way analysis of variance ( p < 0.05). A Tukey post hoc test revealed a statistically significantly higher TWD in morbidly obese (41.5 mg) compared with underweight (25.6 mg, p < 0.05), normal/overweight (28.8 mg, p < 0.05) and obese patients (32.4 mg, p < 0.05). In cohort B, 379 patients were evaluated (9 underweight, 166 normal/overweight, 152 obese, 52 morbidly obese). Overall, 191 patients had a therapeutic INR on discharge (88.9% underweight, 52.4% normal/overweight, 44.1% obese, 55.8% morbidly obese, p = 0.035). Of those, there was a statistically significant difference in TWD ( p = 0.021) with a higher TWD in the morbidly obese (41 mg) compared with underweight patients (24.4 mg, p = 0.017).
Conclusions: Based on the results of this study, morbidly obese patients may require higher TWD to obtain and maintain a therapeutic INR.
Keywords: anticoagulation; atrial fibrillation; obesity; venous thromboembolism; warfarin.
Conflict of interest statement
Figures
Similar articles
-
Impact of body mass index on 90-day warfarin requirements: a retrospective chart review.Ther Adv Cardiovasc Dis. 2021 Jan-Dec;15:17539447211012803. doi: 10.1177/17539447211012803. Ther Adv Cardiovasc Dis. 2021. PMID: 34120532 Free PMC article.
-
Comparison of initial warfarin response in obese patients versus non-obese patients.J Thromb Thrombolysis. 2013 Jul;36(1):96-101. doi: 10.1007/s11239-012-0811-x. J Thromb Thrombolysis. 2013. PMID: 23015280 Clinical Trial.
-
Unfractionated heparin infusion for treatment of venous thromboembolism based on actual body weight without dose capping.Vasc Med. 2020 Feb;25(1):47-54. doi: 10.1177/1358863X19875813. Epub 2019 Oct 18. Vasc Med. 2020. PMID: 31623539
-
Adherence to ESGO guidelines and impact on survival in obese patients with endometrial cancer: a multicentric retrospective study.Int J Gynecol Cancer. 2023 Dec 4;33(12):1950-1956. doi: 10.1136/ijgc-2023-004642. Int J Gynecol Cancer. 2023. PMID: 37788899 Review.
-
Body mass index and pressure injuries risk in hospitalized adult patients: A dose-response analysis.J Tissue Viability. 2024 Aug;33(3):405-411. doi: 10.1016/j.jtv.2024.06.006. Epub 2024 Jun 14. J Tissue Viability. 2024. PMID: 38886143
Cited by
-
Anticoagulation approach in morbid obesity: a comprehensive review on venous thromboembolism management.Front Pharmacol. 2024 Dec 17;15:1457280. doi: 10.3389/fphar.2024.1457280. eCollection 2024. Front Pharmacol. 2024. PMID: 39741630 Free PMC article. Review.
-
Evaluation of Potential Drug Interactions with AiDKlinik® in a Random Population Sample.Integr Pharm Res Pract. 2022 Mar 12;11:61-69. doi: 10.2147/IPRP.S351938. eCollection 2022. Integr Pharm Res Pract. 2022. PMID: 35308067 Free PMC article.
-
Identifying mutation-driven changes in gene functionality that lead to venous thromboembolism.Hum Mutat. 2019 Sep;40(9):1321-1329. doi: 10.1002/humu.23824. Epub 2019 Jul 3. Hum Mutat. 2019. PMID: 31144782 Free PMC article.
-
Evaluation of the impact of body mass index on venous thromboembolism risk factors.PLoS One. 2020 Jul 9;15(7):e0235007. doi: 10.1371/journal.pone.0235007. eCollection 2020. PLoS One. 2020. PMID: 32645000 Free PMC article.
-
A Systematic Review of Polygenic Models for Predicting Drug Outcomes.J Pers Med. 2022 Aug 27;12(9):1394. doi: 10.3390/jpm12091394. J Pers Med. 2022. PMID: 36143179 Free PMC article. Review.
References
-
- Obesity and Overweight. World Health Organization, http://www.who.int/mediacentre/factsheets/fs311/en/ (2017, accessed 5 December 2017).
-
- Bristol-Meyers Squibb. Coumadin® [package insert]. Bristol-Myers Squibb Company: Princeton, New Jersey, 2017.
-
- Marzec JN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017; 69: 2475–2484. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical