Maternal and fetal prognosis of subsequent pregnancy in black African women with peripartum cardiomyopathy
- PMID: 29914408
- PMCID: PMC6006934
- DOI: 10.1186/s12872-018-0856-7
Maternal and fetal prognosis of subsequent pregnancy in black African women with peripartum cardiomyopathy
Abstract
Background: The aim of this study was to describe maternal and fetal outcomes after pregnancy complicated by peripartum cardiomyopathy (PPCM).
Methods: We included women that had subsequent pregnancy (SSP) after PPCM and assessed maternal prognosis and pregnancy outcomes, in-hospital up to one week after discharge. Clinical and echocardiographic data were collected comparing alive and deceased women. Factors associated with pregnancy outcomes were assessed.
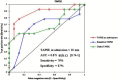
Results: Twenty-nine patients were included, with a mean age of 26.7 ± 4.6 years and a mean gravidity number of 2.3 ± 0.5 of. At the last medical control before subsequent pregnancy, there was no congestive heart failure, the mean left ventricular diastolic diameter (LVDD) was 53 ± 4 mm and the left ventricular ejection fraction (LVEF) was ≥50% in 13 cases (44.8%). Maternal outcomes were marked by 14 deaths (48.3%). Among the factors tested in univariate analysis, LVEF at admission had an excellent receiver-operating characteristic (ROC) curve to predict maternal mortality (AUC = 0.95; 95% CI 0.87-1, p < 0.001), with a cut off value of < 40% (sensitivity = 93% and specificity = 87%). Concerning fetal outcomes, baseline LVEF had the best area under the curve (AUC) to predict abortion or prematurity among all variables (AUC = 0.75; 95% CI 0.58-092, p = 0.003), with a cut-off value of < 50% (sensitivity = 79%, specificity = 67%).
Conclusions: SSP outcomes are still severe in our practice. Maternal mortality remains high and is linked to ventricular systolic function at admission (due to pregnancy), while fetal outcomes are linked to baseline LVEF before pregnancy.
Keywords: Burkina Faso; Peripartum cardiomyopathy; Prognosis; Subsequent pregnancy.
Conflict of interest statement
Ethics approval and consent to participate
We received the approval of the medical ethics committee (comité d’éthique pour la santé au Burkina Faso) and each patient signed an informed consent or affixed their digital loan to participate in the study. Patients applied the fingerprints of his index finger, after having traced it in blue ink.
Consent for publication
Not Applicable.
Competing interests
The authors declares that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. - DOI - PubMed
-
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. - DOI - PubMed
-
- Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, Dec GW, Zucker M, Narula J, Kip K, McNamara DM; IMAC2 Investigators. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail 2012; 18:28–33. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical