Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression
- PMID: 29914512
- PMCID: PMC6006729
- DOI: 10.1186/s12964-018-0221-6
Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression
Abstract
Background: To determine whether adipocyte-derived lipids could be transferred into breast cancer cells and investigate the underlying mechanisms of subsequent lipolysis and fatty acid trafficking in breast cancer cells.
Methods: A Transwell co-culture system was used in which human breast cancer cells were cultured in the absence or presence of differentiated murine 3 T3-L1 adipocytes. Migration/invasion and proliferation abilities were compared between breast cancer cells that were cultivated alone and those co-cultivated with mature adipocytes. The ability of lipolysis in breast cancer cells were measured, as well as the expression of the rate-limiting lipase ATGL and fatty acid transporter FABP5. ATGL and FABP5 were then ablated to investigate their impact on the aggressiveness of breast cancer cells that were surrounded by adipocytes. Further, immunohistochemistry was performed to detect differential expression of ATGL and FABP5 in breast cancer tissue sections.
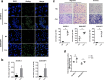
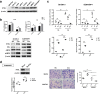
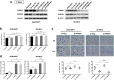
Results: The migration and invasion abilities of cancer cells were significantly enhanced after co-culture with adipocytes, accompanied by elevated lipolysis and expression of ATGL and FABP5. Abrogation of ATGL and FABP5 sharply attenuated the malignancy of co-cultivated breast cancer cells. However, this phenomenon was not observed if a lipid emulsion was added to the culture medium to substitute for adipocytes. Furthermore, epithelial-mesenchymal transaction was induced in co-cultivated breast cancer cells. That may partially due to the stimulation of PPARβ/δ and MAPK, which was resulted from upregulation of FABP5. As evidenced by immunohistochemistry, ATGL and FABP5 also had higher expression levels at the invasive front of the breast tumor, in where the adipocytes abound, compared to the central area in tissue specimens.
Conclusions: Lipid originating from tumor-surrounding adipocytes could be transferred into breast cancer cells. Adipocyte-cancer cell crosstalk rather than lipids alone induced upregulation of lipases and fatty acid transport protein in cancer cells to utilize stored lipids for tumor progression. The increased expression of the key lipase ATGL and intracellular fatty acid trafficking protein FABP5 played crucial roles in this process via fueling or signaling.
Keywords: ATGL; Adipocyte; Aggressiveness; Breast cancer; Crosstalk; FABP5.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Chongqing Medical University for the use of clinical materials for research purposes. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Fatty acid-binding protein 5 (FABP5) promotes lipolysis of lipid droplets, de novo fatty acid (FA) synthesis and activation of nuclear factor-kappa B (NF-κB) signaling in cancer cells.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Sep;1863(9):1057-1067. doi: 10.1016/j.bbalip.2018.06.010. Epub 2018 Jun 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29906613
-
Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells.JCI Insight. 2017 Feb 23;2(4):e87489. doi: 10.1172/jci.insight.87489. JCI Insight. 2017. PMID: 28239646 Free PMC article.
-
Dual regulation of adipose triglyceride lipase by pigment epithelium-derived factor: a novel mechanistic insight into progressive obesity.Mol Cell Endocrinol. 2013 Sep 5;377(1-2):123-34. doi: 10.1016/j.mce.2013.07.001. Epub 2013 Jul 10. Mol Cell Endocrinol. 2013. PMID: 23850519
-
[Adipose triglyceride lipase regulates adipocyte lipolysis].Sheng Li Ke Xue Jin Zhan. 2008 Jan;39(1):10-4. Sheng Li Ke Xue Jin Zhan. 2008. PMID: 18357681 Review. Chinese.
-
Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease.J Mol Endocrinol. 2014 Jun;52(3):R199-222. doi: 10.1530/JME-13-0277. Epub 2014 Feb 27. J Mol Endocrinol. 2014. PMID: 24577718 Review.
Cited by
-
Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics.J Hematol Oncol. 2023 Sep 12;16(1):103. doi: 10.1186/s13045-023-01498-2. J Hematol Oncol. 2023. PMID: 37700339 Free PMC article. Review.
-
Revolutionizing breast cancer treatment: Harnessing the related mechanisms and drugs for regulated cell death (Review).Int J Oncol. 2024 May;64(5):46. doi: 10.3892/ijo.2024.5634. Epub 2024 Mar 8. Int J Oncol. 2024. PMID: 38456493 Free PMC article.
-
Metabolic Adaptations in Cancer Progression: Optimization Strategies and Therapeutic Targets.Cancers (Basel). 2025 Jul 15;17(14):2341. doi: 10.3390/cancers17142341. Cancers (Basel). 2025. PMID: 40723225 Free PMC article. Review.
-
The RAGE/multiligand axis: a new actor in tumor biology.Biosci Rep. 2022 Jul 29;42(7):BSR20220395. doi: 10.1042/BSR20220395. Biosci Rep. 2022. PMID: 35727208 Free PMC article. Review.
-
Tumour fatty acid metabolism in the context of therapy resistance and obesity.Nat Rev Cancer. 2021 Dec;21(12):753-766. doi: 10.1038/s41568-021-00388-4. Epub 2021 Aug 20. Nat Rev Cancer. 2021. PMID: 34417571 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

