Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial
- PMID: 29914530
- PMCID: PMC6006766
- DOI: 10.1186/s13054-018-2086-x
Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial
Abstract
Background: There is increasing interest in the timely administration of concentrated sources of fibrinogen to patients with major traumatic bleeding. Following evaluation of early cryoprecipitate in the CRYOSTAT 1 trial, we explored the use of fibrinogen concentrate, which may have advantages of more rapid administration in acute haemorrhage. The aims of this pragmatic study were to assess the feasibility of fibrinogen concentrate administration within 45 minutes of hospital admission and to quantify efficacy in maintaining fibrinogen levels ≥ 2 g/L during active haemorrhage.
Methods: We conducted a blinded, randomised, placebo-controlled trial at five UK major trauma centres with adult trauma patients with active bleeding who required activation of the major haemorrhage protocol. Participants were randomised to standard major haemorrhage therapy plus 6 g of fibrinogen concentrate or placebo.
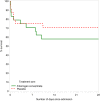
Results: Twenty-seven of 39 participants (69%; 95% CI, 52-83%) across both arms received the study intervention within 45 minutes of admission. There was some evidence of a difference in the proportion of participants with fibrinogen levels ≥ 2 g/L between arms (p = 0.10). Fibrinogen levels in the fibrinogen concentrate (FgC) arm rose by a mean of 0.9 g/L (SD, 0.5) compared with a reduction of 0.2 g/L (SD, 0.5) in the placebo arm and were significantly higher in the FgC arm (p < 0.0001) at 2 hours. Fibrinogen levels were not different at day 7. Transfusion use and thromboembolic events were similar between arms. All-cause mortality at 28 days was 35.5% (95% CI, 23.8-50.8%) overall, with no difference between arms.
Conclusions: In this trial, early delivery of fibrinogen concentrate within 45 minutes of admission was not feasible. Although evidence points to a key role for fibrinogen in the treatment of major bleeding, researchers need to recognise the challenges of timely delivery in the emergency setting. Future studies must explore barriers to rapid fibrinogen therapy, focusing on methods to reduce time to randomisation, using 'off-the-shelf' fibrinogen therapies (such as extended shelf-life cryoprecipitate held in the emergency department or fibrinogen concentrates with very rapid reconstitution times) and limiting the need for coagulation test-based transfusion triggers.
Trial registration: ISRCTN67540073 . Registered on 5 August 2015.
Keywords: Cryoprecipitate; Fibrinogen replacement therapy; Haemorrhagic shock; Multiple trauma; Transfusion.
Conflict of interest statement
Ethics approval and consent to participate
An emergency waiver with an independent agreement process was used. The protocol and consent process were approved by the NRES Committee South Central Oxford C (15/S3/0316) and the MHRA (25224/0003/001-0001). Written informed consent was sought from the participant as soon as practically possible after study entry for continuation in the trial. If the participant did not regain capacity, agreement was sought from the participant’s next of kin or other appropriate representative.
Competing interests
NC has received support for conference attendance from CSL Behring and is a consultant for LFB. RD has received consultancy fees from LFB. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical