Local and global influences on protein turnover in neurons and glia
- PMID: 29914620
- PMCID: PMC6008053
- DOI: 10.7554/eLife.34202
Local and global influences on protein turnover in neurons and glia
Abstract
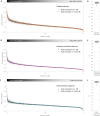
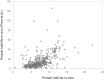
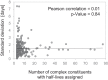
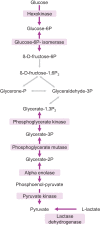
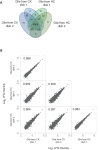
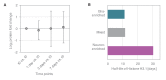
Regulation of protein turnover allows cells to react to their environment and maintain homeostasis. Proteins can show different turnover rates in different tissue, but little is known about protein turnover in different brain cell types. We used dynamic SILAC to determine half-lives of over 5100 proteins in rat primary hippocampal cultures as well as in neuron-enriched and glia-enriched cultures ranging from <1 to >20 days. In contrast to synaptic proteins, membrane proteins were relatively shorter-lived and mitochondrial proteins were longer-lived compared to the population. Half-lives also correlate with protein functions and the dynamics of the complexes they are incorporated in. Proteins in glia possessed shorter half-lives than the same proteins in neurons. The presence of glia sped up or slowed down the turnover of neuronal proteins. Our results demonstrate that both the cell-type of origin as well as the nature of the extracellular environment have potent influences on protein turnover.
Keywords: Hippocampus; Protein turnover; Proteomics; cell biology; neuron-glia interactions; neuroscience; rat.
© 2018, Dörrbaum et al.
Conflict of interest statement
AD, LK, JL, ES No competing interests declared
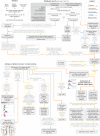
Figures










Similar articles
-
Dynamic SILAC to Determine Protein Turnover in Neurons and Glia.Methods Mol Biol. 2023;2603:1-17. doi: 10.1007/978-1-0716-2863-8_1. Methods Mol Biol. 2023. PMID: 36370266
-
TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development.Glia. 2007 Aug 1;55(10):1023-33. doi: 10.1002/glia.20522. Glia. 2007. PMID: 17549683
-
Pgrmc1/BDNF Signaling Plays a Critical Role in Mediating Glia-Neuron Cross Talk.Endocrinology. 2016 May;157(5):2067-79. doi: 10.1210/en.2015-1610. Epub 2016 Mar 18. Endocrinology. 2016. PMID: 26990062 Free PMC article.
-
Chemistry and cell biology of neuron- and glia-specific proteins.Arch Histol Cytol. 1989;52 Suppl:25-32. doi: 10.1679/aohc.52.suppl_25. Arch Histol Cytol. 1989. PMID: 2510787 Review.
-
Calcium waves rule and divide radial glia.Neuron. 2004 Sep 2;43(5):599-601. doi: 10.1016/j.neuron.2004.08.030. Neuron. 2004. PMID: 15339639 Review.
Cited by
-
Macroautophagy and Mitophagy in Neurodegenerative Disorders: Focus on Therapeutic Interventions.Biomedicines. 2021 Nov 5;9(11):1625. doi: 10.3390/biomedicines9111625. Biomedicines. 2021. PMID: 34829854 Free PMC article. Review.
-
Control of membrane protein homeostasis by a chaperone-like glial cell adhesion molecule at multiple subcellular locations.Sci Rep. 2021 Sep 16;11(1):18435. doi: 10.1038/s41598-021-97777-4. Sci Rep. 2021. PMID: 34531445 Free PMC article.
-
DELTA: a method for brain-wide measurement of synaptic protein turnover reveals localized plasticity during learning.Nat Neurosci. 2025 May;28(5):1089-1098. doi: 10.1038/s41593-025-01923-4. Epub 2025 Mar 31. Nat Neurosci. 2025. PMID: 40164741 Free PMC article.
-
Aere perennius: how chromatin fidelity is maintained and lost in disease.NAR Mol Med. 2025 Jul 22;2(3):ugaf026. doi: 10.1093/narmme/ugaf026. eCollection 2025 Jul. NAR Mol Med. 2025. PMID: 40809411 Free PMC article. Review.
-
Fructose Removal from the Diet Reverses Inflammation, Mitochondrial Dysfunction, and Oxidative Stress in Hippocampus.Antioxidants (Basel). 2021 Mar 20;10(3):487. doi: 10.3390/antiox10030487. Antioxidants (Basel). 2021. PMID: 33804637 Free PMC article.
References
-
- Alvarez-Castelao B, Schanzenbächer CT, Hanus C, Glock C, Tom Dieck S, Dörrbaum AR, Bartnik I, Nassim-Assir B, Ciirdaeva E, Mueller A, Dieterich DC, Tirrell DA, Langer JD, Schuman EM. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nature Biotechnology. 2017;35:1196. doi: 10.1038/nbt.4016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

