Role of complement C5a and histones in septic cardiomyopathy
- PMID: 29914696
- PMCID: PMC6139045
- DOI: 10.1016/j.molimm.2018.06.006
Role of complement C5a and histones in septic cardiomyopathy
Abstract
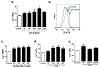
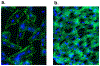
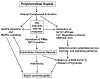
Polymicrobial sepsis (after cecal ligation and puncture, CLP) causes robust complement activation with release of C5a. Many adverse events develop thereafter and will be discussed in this review article. Activation of complement system results in generation of C5a which interacts with its receptors (C5aR1, C5aR2). This leads to a series of harmful events, some of which are connected to the cardiomyopathy of sepsis, resulting in defective action potentials in cardiomyocytes (CMs), activation of the NLRP3 inflammasome in CMs and the appearance of extracellular histones, likely arising from activated neutrophils which form neutrophil extracellular traps (NETs). These events are associated with activation of mitogen-activated protein kinases (MAPKs) in CMs. The ensuing release of histones results in defective action potentials in CMs and reduced levels of [Ca2+]i-regulatory enzymes including sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2) and Na+/Ca2+ exchanger (NCX) as well as Na+/K+-ATPase in CMs. There is also evidence that CLP causes release of IL-1β via activation of the NLRP3 inflammasome in CMs of septic hearts or in CMs incubated in vitro with C5a. Many of these events occur after in vivo or in vitro contact of CMs with histones. Together, these data emphasize the role of complement (C5a) and C5a receptors (C5aR1, C5aR2), as well as extracellular histones in events that lead to cardiac dysfunction of sepsis (septic cardiomyopathy).
Keywords: Cecal ligation and puncture (CLP); Intracellular calcium ([Ca(2+)]i); NLRP3 inflammasome; Na+/Ca(2+)exchanger (NCX); Na+/K+-ATPase; Sarco/endoplasmic reticulum Ca(2+)-ATPase (SERCA2).
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abbate A, et al. 2010. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail 12(4):319–22. - PubMed
-
- Adib-Conquy M, et al. 2007. Increased plasma levels of soluble triggering receptor expressed on myeloid cells 1 and procalcitonin after cardiac surgery and cardiac arrest without infection. Shock 28(4):406–10. - PubMed
-
- Alhamdi Y, et al. 2015. Circulating Histones Are Major Mediators of Cardiac Injury in Patients With Sepsis. Crit Care Med 43(10):2094–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

