Peripheral and Central Nutrient Sensing Underlying Appetite Regulation
- PMID: 29914721
- PMCID: PMC6064385
- DOI: 10.1016/j.tins.2018.05.003
Peripheral and Central Nutrient Sensing Underlying Appetite Regulation
Abstract
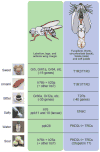
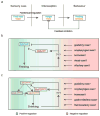
The precise regulation of fluid and energy homeostasis is essential for survival. It is well appreciated that ingestive behaviors are tightly regulated by both peripheral sensory inputs and central appetite signals. With recent neurogenetic technologies, considerable progress has been made in our understanding of basic taste qualities, the molecular and/or cellular basis of taste sensing, and the central circuits for thirst and hunger. In this review, we first highlight the functional similarities and differences between mammalian and invertebrate taste processing. We then discuss how central thirst and hunger signals interact with peripheral sensory signals to regulate ingestive behaviors. We finally indicate some of the directions for future research.
Keywords: hunger; sensory valence; taste; thirst; top-down regulation.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

References
-
- Gizowski C, Bourque CW. The neural basis of homeostatic and anticipatory thirst. Nature Reviews Nephrology. 2017 nrneph. 2017.2149. - PubMed
-
- Saper CB, et al. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. - PubMed
-
- Weigle DS. Appetite and the regulation of body composition. The FASEB Journal. 1994;8:302–310. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

