Thailandamide, a Fatty Acid Synthesis Antibiotic That Is Coexpressed with a Resistant Target Gene
- PMID: 29914944
- PMCID: PMC6125523
- DOI: 10.1128/AAC.00463-18
Thailandamide, a Fatty Acid Synthesis Antibiotic That Is Coexpressed with a Resistant Target Gene
Abstract
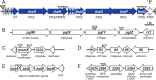
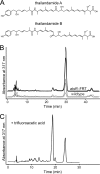
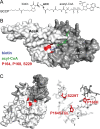
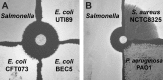
Microbes encode many uncharacterized gene clusters that may produce antibiotics and other bioactive small molecules. Methods for activating these genes are needed to explore their biosynthetic potential. A transposon containing an inducible promoter was randomly inserted into the genome of the soil bacterium Burkholderia thailandensis to induce antibiotic expression. This screen identified the polyketide/nonribosomal peptide thailandamide as an antibiotic and discovered its regulator, AtsR. Mutants of Salmonella resistant to thailandamide had mutations in the accA gene for acetyl coenzyme A (acetyl-CoA) carboxylase, which is one of the first enzymes in the fatty acid synthesis pathway. A second copy of accA in the thailandamide synthesis gene cluster keeps B. thailandensis resistant to its own antibiotic. These genetic techniques will likely be powerful tools for discovering other unusual antibiotics.
Keywords: Burkholderia thailandensis; acetyl-CoA carboxylase; antimicrobial; global regulator; polyketide.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- CDC. 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
-
- Palleroni NJ. 2005. Burkholderia, p 575–600. In Garrity GM, Brenner DJ, Krieg NR, Staley JT (ed), Bergey's manual of systematic bacteriology, vol 2 Springer, New York, NY.
-
- Walsh C. 2003. Antibiotics: actions, origins, resistance. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

