Pharmacokinetics, Safety, and Tolerability of Single and Multiple Doses of Relebactam, a β-Lactamase Inhibitor, in Combination with Imipenem and Cilastatin in Healthy Participants
- PMID: 29914955
- PMCID: PMC6125551
- DOI: 10.1128/AAC.00280-18
Pharmacokinetics, Safety, and Tolerability of Single and Multiple Doses of Relebactam, a β-Lactamase Inhibitor, in Combination with Imipenem and Cilastatin in Healthy Participants
Erratum in
-
Erratum for Rhee et al., "Pharmacokinetics, Safety, and Tolerability of Single and Multiple Doses of Relebactam, a β-Lactamase Inhibitor, in Combination with Imipenem and Cilastatin in Healthy Participants".Antimicrob Agents Chemother. 2018 Nov 26;62(12):e02197-18. doi: 10.1128/AAC.02197-18. Print 2018 Dec. Antimicrob Agents Chemother. 2018. PMID: 30478180 Free PMC article. No abstract available.
Abstract
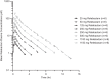
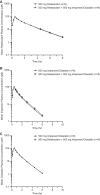
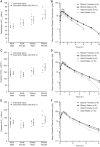
Relebactam is a novel class A and C β-lactamase inhibitor that is being developed in combination with imipenem-cilastatin for the treatment of serious infections with Gram-negative bacteria. Here we report on two phase 1 randomized, double-blind, placebo-controlled pharmacokinetics, safety, and tolerability studies of relebactam administered with or without imipenem-cilastatin to healthy participants: (i) a single-dose (25 to 1,150 mg) and multiple-dose (50 to 625 mg every 6 h [q6h] for 7 to 14 days) escalation study with men and (ii) a single-dose (125 mg) study with women and elderly individuals. Following single- or multiple-dose intravenous administration over 30 min, plasma relebactam concentrations declined biexponentially, with a terminal half-life (t1/2) ranging from 1.35 to 1.85 h independently of the dose. Exposures increased in a dose-proportional manner across the dose range. No clinically significant differences in pharmacokinetics between men and women, or between adult and elderly participants, were observed. Urine pharmacokinetics demonstrated that urinary excretion is the major route of relebactam elimination. No drug-drug interaction between relebactam and imipenem-cilastatin was observed, and the observed t1/2 values for relebactam, imipenem, and cilastatin were comparable, thus supporting coadministration. Relebactam administered alone or in combination with imipenem-cilastatin was well tolerated across the dose ranges studied. No serious adverse events or deaths were reported. The pharmacokinetic profile and favorable safety results supported q6h dosing of relebactam with imipenem-cilastatin in clinical treatment trials.
Keywords: pharmacokinetics; relebactam; β-lactamase inhibitor.
Copyright © 2018 Rhee et al.
Figures

References
-
- Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. 2017. Antimicrobial susceptibility of Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Asia-Pacific countries: SMART 2013–2015. J Med Microbiol 66:61–69. doi: 10.1099/jmm.0.000421. - DOI - PubMed
-
- Karlowsky JA, Hoban DJ, Hackel MA, Lob SH, Sahm DF. 2017. Resistance among Gram-negative ESKAPE pathogens isolated from hospitalized patients with intra-abdominal and urinary tract infections in Latin American countries: SMART 2013–2015. Braz J Infect Dis 21:343–348. doi: 10.1016/j.bjid.2017.03.006. - DOI - PMC - PubMed
-
- Weiner LM, Fridkin SK, Aponte-Torres Z, Avery L, Coffin N, Dudeck MA, Edwards JR, Jernigan JA, Konnor R, Soe MM, Peterson K, McDonald LC. 2016. Vital signs: preventing antibiotic-resistant infections in hospitals—United States, 2014. MMWR Morb Mortal Wkly Rep 65:235–241. doi: 10.15585/mmwr.mm6509e1. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

