Long noncoding RNA MIAT regulates apoptosis and the apoptotic response to chemotherapeutic agents in breast cancer cell lines
- PMID: 29914974
- PMCID: PMC6435567
- DOI: 10.1042/BSR20180704
Long noncoding RNA MIAT regulates apoptosis and the apoptotic response to chemotherapeutic agents in breast cancer cell lines
Abstract
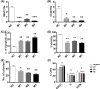
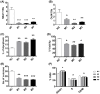
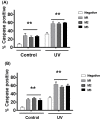
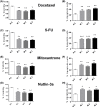
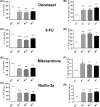
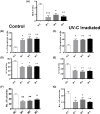
The long noncoding RNA myocardial infarction associated transcript (MIAT) is involved in a number of diseases, including myocardial infarction and diabetic retinopathy. Emerging evidence suggests that MIAT expression levels are increased in different type of cancers, including breast cancer. In the present study, we further evaluated the role of MIAT in breast cancer and investigated the consequences of its silencing on breast cancer response to chemotherapeutic agents. Expression levels of MIAT mRNA in breast cancer were determined using TissueScan™ Breast Cancer cDNA Arrays. Breast cancer cell lines were transfected with MIAT specific siRNAs, with silencing confirmed using RT-qPCR and the effects on breast cancer cell survival and response to different apoptotic stimuli determined. MIAT transcript levels were significantly elevated in breast cancer samples. Such increase was specific to the early stages of the disease, ER, PR +ve, HER -ve, and triple negative breast cancer samples. Silencing of MIAT induced growth arrest and increased basal apoptosis. Reduced levels of MIAT augmented the apoptotic response of breast cancer cells to a wide range of apoptotic stimuli. Our results also showed that MIAT down-regulation was associated with a decrease in OCT4 mRNA, suggesting the existence of a MIAT/OCT4 regulatory loop, similar to that observed in malignant mature B cells. Taken together with the recent demonstration of oncogene characteristics, our observations suggest that MIAT plays an important role in breast tumorigenesis. Strategies to decrease MIAT expression levels may improve sensitivity to therapy in breast cancer by enhancing the apoptotic responses to conventional chemotherapies.
Keywords: Apoptosis; Breast; Cancer; Chemotherapy; MIAT; OCT4.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

