Simultaneous activation of innate and adaptive immunity participates in the development of renal injury in a model of heavy proteinuria
- PMID: 29914975
- PMCID: PMC6043717
- DOI: 10.1042/BSR20180762
Simultaneous activation of innate and adaptive immunity participates in the development of renal injury in a model of heavy proteinuria
Abstract
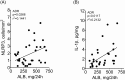
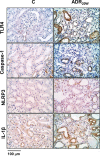
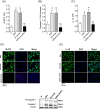
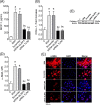
Protein overload of proximal tubular cells (PTCs) can promote interstitial injury by unclear mechanisms that may involve activation of innate immunity. We investigated whether prolonged exposure of tubular cells to high protein concentrations stimulates innate immunity, triggering progressive interstitial inflammation and renal injury, and whether specific inhibition of innate or adaptive immunity would provide renoprotection in an established model of massive proteinuria, adriamycin nephropathy (ADR). Adult male Munich-Wistar rats received a single dose of ADR (5 mg/kg, iv), being followed for 2, 4, or 20 weeks. Massive albuminuria was associated with early activation of both the NF-κB and NLRP3 innate immunity pathways, whose intensity correlated strongly with the density of lymphocyte infiltration. In addition, ADR rats exhibited clear signs of renal oxidative stress. Twenty weeks after ADR administration, marked interstitial fibrosis, glomerulosclerosis, and renal functional loss were observed. Administration of mycophenolate mofetil (MMF), 10 mg/kg/day, prevented activation of both innate and adaptive immunity, as well as renal oxidative stress and renal fibrosis. Moreover, MMF treatment was associated with shifting of M from the M1 to the M2 phenotype. In cultivated NRK52-E cells, excess albumin increased the protein content of Toll-like receptor (TLR) 4 (TLR4), NLRP3, MCP-1, IL6, IL-1β, Caspase-1, α-actin, and collagen-1. Silencing of TLR4 and/or NLRP3 mRNA abrogated this proinflammatory/profibrotic behavior. Simultaneous activation of innate and adaptive immunity may be key to the development of renal injury in heavy proteinuric disease. Inhibition of specific components of innate and/or adaptive immunity may be the basis for future strategies to prevent chronic kidney disease (CKD) in this setting.
Keywords: NF-κB system; NLRP3 inflammasome; adaptive immunity; chronic kidney disease; proteinuria.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

