Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation
- PMID: 29915060
- PMCID: PMC6142213
- DOI: 10.1073/pnas.1715932115
Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation
Abstract
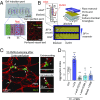
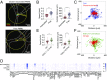
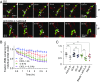
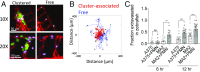
Systemic inflammation occurring around the course of tumor progression and treatment are often correlated with adverse oncological outcomes. As such, it is suspected that neutrophils, the first line of defense against infection, may play important roles in linking inflammation and metastatic seeding. To decipher the dynamic roles of inflamed neutrophils during hematogenous dissemination, we employ a multiplexed microfluidic model of the human microvasculature enabling physiologically relevant transport of circulating cells combined with real-time, high spatial resolution observation of heterotypic cell-cell interactions. LPS-stimulated neutrophils (PMNs) and tumor cells (TCs) form heterotypic aggregates under flow, and arrest due to both mechanical trapping and neutrophil-endothelial adhesions. Surprisingly, PMNs are not static following aggregation, but exhibit a confined migration pattern near TC-PMN clusters. We discover that PMNs are chemotactically confined by self-secreted IL-8 and tumor-derived CXCL-1, which are immobilized by the endothelial glycocalyx. This results in significant neutrophil sequestration with arrested tumor cells, leading to the spatial localization of neutrophil-derived IL-8, which also contributes to increasing the extravasation potential of adjacent tumor cells through modulation of the endothelial barrier. Strikingly similar migration patterns and extravasation behaviors were also observed in an in vivo zebrafish model upon PMN-tumor cell coinjection into the embryo vasculature. These insights into the temporal dynamics of intravascular tumor-PMN interactions elucidate the mechanisms through which inflamed neutrophils can exert proextravasation effects at the distant metastatic site.
Keywords: cell migration; extravasation; inflammation; metastasis; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

