Hyperstimulation of CaSR in human MSCs by biomimetic apatite inhibits endochondral ossification via temporal down-regulation of PTH1R
- PMID: 29915064
- PMCID: PMC6142224
- DOI: 10.1073/pnas.1805159115
Hyperstimulation of CaSR in human MSCs by biomimetic apatite inhibits endochondral ossification via temporal down-regulation of PTH1R
Abstract
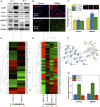
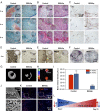
In adult bone injuries, periosteum-derived mesenchymal stem/stromal cells (MSCs) form bone via endochondral ossification (EO), whereas those from bone marrow (BM)/endosteum form bone primarily through intramembranous ossification (IMO). We hypothesized that this phenomenon is influenced by the proximity of MSCs residing in the BM to the trabecular bone microenvironment. Herein, we investigated the impact of the bone mineral phase on human BM-derived MSCs' choice of ossification pathway, using a biomimetic bone-like hydroxyapatite (BBHAp) interface. BBHAp induced hyperstimulation of extracellular calcium-sensing receptor (CaSR) and temporal down-regulation of parathyroid hormone 1 receptor (PTH1R), leading to inhibition of chondrogenic differentiation of MSCs even in the presence of chondroinductive factors, such as transforming growth factor-β1 (TGF-β1). Interestingly rescuing PTH1R expression using human PTH fragment (1-34) partially restored chondrogenesis in the BBHAp environment. In vivo studies in an ectopic site revealed that the BBHAp interface inhibits EO and strictly promotes IMO. Furthermore, CaSR knockdown (CaSR KD) disrupted the bone-forming potential of MSCs irrespective of the absence or presence of the BBHAp interface. Our findings confirm the expression of CaSR in human BM-derived MSCs and unravel a prominent role for the interplay between CaSR and PTH1R in regulating MSC fate and the choice of pathway for bone formation.
Keywords: Wnt signaling; bone remodeling; calcium phosphate; regenerative medicine; stem cell niche.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Karaplis AC. Embryonic development of bone and regulation of intramembranous and endochondral bone formation. Princ Bone Biol. 2008;1:53–84.
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. - PubMed
-
- Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

