Francisella marina sp. nov., Etiologic Agent of Systemic Disease in Cultured Spotted Rose Snapper (Lutjanus guttatus) in Central America
- PMID: 29915103
- PMCID: PMC6070750
- DOI: 10.1128/AEM.00144-18
Francisella marina sp. nov., Etiologic Agent of Systemic Disease in Cultured Spotted Rose Snapper (Lutjanus guttatus) in Central America
Abstract
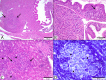
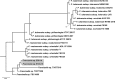
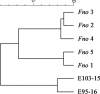
Historically, piscine francisellosis in various warm-, temperate-, and cold-water fish hosts has been attributed to Francisella noatunensis From 2015 to 2016, an undescribed Francisella sp. was recovered during mortality events in cultured spotted rose snapper (Lutjanus guttatus) off the Pacific coast of Central America. Despite high mortality and emaciation, limited gross findings were observed in affected fish. Histological examination revealed multifocal granulomatous lesions, with the presence of numerous small, pleomorphic coccobacilli, predominantly in the peritoneum, spleen, kidneys, liver, pancreas, heart, and intestine. Sequencing of an ∼1,400-bp fragment of the 16S rRNA gene demonstrated these isolates to be most similar (99.9% identity) to Francisella sp. isolate TX077308 cultured from seawater in the Gulf of Mexico, while sharing <99% similarity to other Fransicella spp. Biochemical analysis, multilocus sequence comparisons of select housekeeping genes, repetitive extragenic palindromic PCR fingerprinting, matrix-assisted laser desorption ionization-time of flight mass spectrometry, and fatty acid methyl ester analysis revealed marked differences between these isolates and other described members of the genus. Koch's postulates were fulfilled by experimental intracoelomic injection and immersion trials using Nile (Oreochromis niloticus) and blue (Oreochromis aureus) tilapia. Based on observed phenotypic and genotypic differences from recognized Francisella spp., the name Francisellamarina sp. nov. (NRRL B-65518) is proposed to accommodate these novel strains.IMPORTANCE Finfish aquaculture is the fastest growing global food production sector. Infectious disease, particularly emergent pathogens, pose a significant threat to established and nascent aquaculture industries worldwide. Herein, we characterize a novel pathogen isolated from mortality events in cultured spotted rose snapper in Central America. The bacteria recovered from these outbreaks were genetically and phenotypically dissimilar from other known Francisella spp. from fish, representing a previously unrecognized member of the genus Francisella, for which the name Francisella marina sp. nov. is proposed.
Keywords: Francisella; aquaculture; fish pathogens; snapper.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- Food Agriculture Organization, United Nations. 2011. The state of world fisheries and aquaculture 2010. Food Agriculture Organization, United Nations, Rome, Italy.
-
- Krkosek M. 2010. Host density thresholds and disease control for fisheries and aquaculture. Aquac Environ Interact 1:21–32. doi: 10.3354/aei0004. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

