Efficacy and Safety of Ticagrelor in Relation to Aspirin Use Within the Week Before Randomization in the SOCRATES Trial
- PMID: 29915123
- PMCID: PMC6019568
- DOI: 10.1161/STROKEAHA.118.020553
Efficacy and Safety of Ticagrelor in Relation to Aspirin Use Within the Week Before Randomization in the SOCRATES Trial
Abstract
Background and purpose: SOCRATES (Acute Stroke or Transient Ischemic Attack Treated With Aspirin or Ticagrelor and Patient Outcomes), comparing ticagrelor with aspirin in patients with acute cerebral ischemia, found a nonsignificant 11% relative risk reduction for stroke, myocardial infarction, or death (P=0.07). Aspirin intake before randomization could enhance the effect of ticagrelor by conferring dual antiplatelet effect during a high-risk period for subsequent stroke. Therefore, we explored the efficacy and safety of ticagrelor versus aspirin in the patients who received any aspirin the week before randomization.
Methods: A prespecified subgroup analysis in SOCRATES (n=13 199), randomizing patients with acute ischemic stroke (National Institutes of Health Stroke Scale score of ≤5) or transient ischemic attack (ABCD2 score of ≥4) to 90-day treatment with ticagrelor or aspirin. Patients in the prior-aspirin group had received any aspirin within the week before randomization. Primary end point was time to stroke, myocardial infarction, or death. Safety end point was PLATO (Study of Platelet Inhibition and Patient Outcomes) major bleeding.
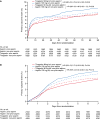
Results: The 4232 patients in the prior-aspirin group were older, had more vascular risk factors, and vascular disease than the other patients. In the prior-aspirin group, the primary end point occurred in 138/2130 (6.5%) of patients on ticagrelor and in 177/2102 (8.3%) on aspirin (hazard ratio, 0.76; 95% confidence interval, 0.61-0.95; P=0.02); in patients with no prior-aspirin usage an event occurred in 304/4459 (6.9%) and 320/4508 (7.1%) on ticagrelor and aspirin, respectively (hazard ratio, 0.96; 95% confidence interval, 0.82-1.12; P=0.59). The treatment-by-prior-aspirin interaction was not statistically significant (P=0.10). In the prior-aspirin group, major bleeding occurred in 0.7% and 0.4% of patients on ticagrelor and aspirin, respectively (hazard ratio, 1.58; 95% confidence interval, 0.68-3.65; P=0.28).
Conclusions: In this secondary analysis from SOCRATES, fewer primary end points occurred on ticagrelor treatment than on aspirin in patients receiving aspirin before randomization, but there was no significant treatment-by-prior-aspirin interaction. A new study will investigate the benefit-risk of combining ticagrelor and aspirin in patients with acute cerebral ischemia (URL: https://www.clinicaltrials.gov. Unique identifier: NCT03354429).
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT01994720.
Keywords: aspirin; platelet aggregation inhibitors; stroke; ticagrelor; transient ischemic attack.
© 2018 American Heart Association, Inc.
Figures
References
-
- Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, et al. TIAregistry.org Investigators One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981. - DOI - PubMed
-
- CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomized placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. - PubMed
-
- International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

