Known structures and unknown mechanisms of TMEM16 scramblases and channels
- PMID: 29915161
- PMCID: PMC6028493
- DOI: 10.1085/jgp.201711957
Known structures and unknown mechanisms of TMEM16 scramblases and channels
Abstract
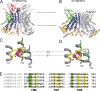
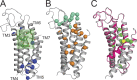
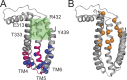
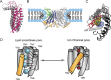
The TMEM16 family of membrane proteins is composed of both Ca2+-gated Cl- channels and Ca2+-dependent phospholipid scramblases. The functional diversity of TMEM16s underlies their involvement in numerous signal transduction pathways that connect changes in cytosolic Ca2+ levels to cellular signaling networks. Indeed, defects in the function of several TMEM16s cause a variety of genetic disorders, highlighting their fundamental pathophysiological importance. Here, we review how our mechanistic understanding of TMEM16 function has been shaped by recent functional and structural work. Remarkably, the recent determination of near-atomic-resolution structures of TMEM16 proteins of both functional persuasions has revealed how relatively minimal rearrangements in the substrate translocation pathway are sufficient to precipitate the dramatic functional differences that characterize the family. These structures, when interpreted in the light of extensive functional analysis, point to an unusual mechanism for Ca2+-dependent activation of TMEM16 proteins in which substrate permeation is regulated by a combination of conformational rearrangements and electrostatics. These breakthroughs pave the way to elucidate the mechanistic bases of ion and lipid transport by the TMEM16 proteins and unravel the molecular links between these transport activities and their function in human pathophysiology.
© 2018 Falzone et al.
Figures

References
-
- Almaça J., Tian Y., Aldehni F., Ousingsawat J., Kongsuphol P., Rock J.R., Harfe B.D., Schreiber R., and Kunzelmann K.. 2009. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 284:28571–28578. 10.1074/jbc.M109.010074 - DOI - PMC - PubMed
-
- Baig A.A., Haining E.J., Geuss E., Beck S., Swieringa F., Wanitchakool P., Schuhmann M.K., Stegner D., Kunzelmann K., Kleinschnitz C., et al. . 2016. TMEM16F-mediated platelet membrane phospholipid scrambling is critical for hemostasis and thrombosis but not thromboinflammation in mice-brief report. Arterioscler. Thromb. Vasc. Biol. 36:2152–2157. 10.1161/ATVBAHA.116.307727 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

