A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom
- PMID: 29915191
- PMCID: PMC6189132
- DOI: 10.1038/s41433-018-0144-x
A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom
Abstract
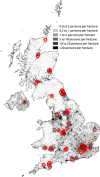
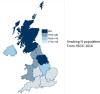
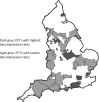
This prospective British Ophthalmological Surveillance Unit (BOSU) study on dysthyroid optic neuropathy (DON) determines the incidence, presenting features and management throughout the UK. New cases were identified through the BOSU yellow card and an initial questionnaire and a subsequent 9-month follow-up questionnaire were posted out. From August 2015 to August 2016 DON was reported in 49 patients with 71 eyes affected, 22 patients had bilateral DON. The most common presenting symptom was blurred vision (83%) and the most common examination finding was upgaze restriction (85%). 85% of patients were initially treated with 3 days of either 1 g or 500 mg intravenous methyl prednisolone. We received 25 follow-up questionnaires (51% of the initial cohort) with 38 eyes treated for DON and 13 bilateral cases. The average steroid dose over 9 months was 4.5 g and 47% of patients had a surgical orbital decompression. The mean visual acuity gain after 9 months of follow-up for all patients was 0.25 LogMAR. The mean visual acuity gain after just medical therapy was 0.25 LogMAR and after both medical therapy and orbital decompression it was 0.24 LogMAR. In conclusion, the incidence of DON in the UK from this study is 0.75 per million population per annum. The majority of patients are treated with initial medical therapy and almost half of all patients subsequently went on to have an orbital decompression. With either medical therapy or medical and surgical therapy, vision can improve in patients with DON.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
Comment on: A British Ophthalmic Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom.Eye (Lond). 2019 Feb;33(2):327-342. doi: 10.1038/s41433-018-0303-0. Epub 2018 Dec 12. Eye (Lond). 2019. PMID: 30542064 Free PMC article. No abstract available.
References
-
- Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98:1443–9. doi: 10.1210/jc.2012-3873. - DOI - PubMed
-
- Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Currò N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marinò M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, Wiersinga WM. European Group on Graves’ Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Thyroid. 2008;18:281–2. doi: 10.1089/thy.2007.0315. - DOI - PubMed
-
- Perros, et al. A questionnaire survey on the management of Graves’ orbitopathy in Europe. Eur J Endocrinol August. 2006;1:155207–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical