The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism
- PMID: 29915192
- PMCID: PMC6035776
- DOI: 10.1038/s41593-018-0167-4
The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism
Abstract
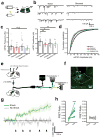
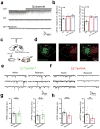
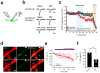
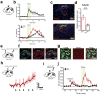
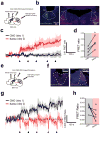
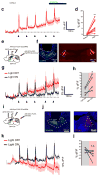
The paraventricular nucleus of the thalamus (PVT) is increasingly being recognized as a critical node linking stress detection to the emergence of adaptive behavioral responses to stress. However, despite growing evidence implicating the PVT in stress processing, the neural mechanisms by which stress impacts PVT neurocircuitry and promotes stressed states remain unknown. Here we show that stress exposure drives a rapid and persistent reduction of inhibitory transmission onto projection neurons of the posterior PVT (pPVT). This stress-induced disinhibition of the pPVT was associated with a locus coeruleus-mediated rise in the extracellular concentration of dopamine in the midline thalamus, required the function of dopamine D2 receptors on PVT neurons, and increased sensitivity to stress. Our findings define the locus coeruleus as an important modulator of PVT function: by controlling the inhibitory tone of the pPVT, it modulates the excitability of pPVT projection neurons and controls stress responsivity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Stressing the other paraventricular nucleus.Nat Neurosci. 2018 Jul;21(7):901-902. doi: 10.1038/s41593-018-0178-1. Nat Neurosci. 2018. PMID: 29942038 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

